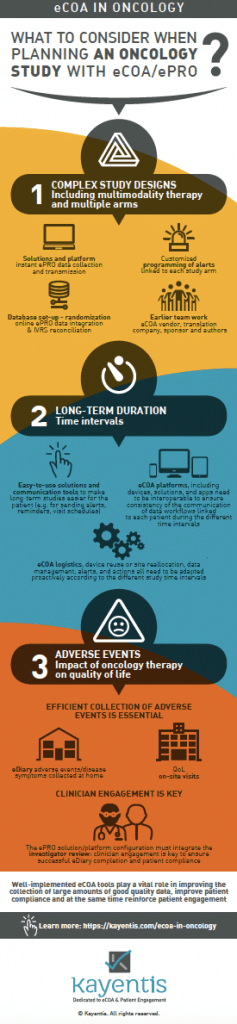
Infography: planning an oncology study with eCOA/ePRO
The FDA has provided guidance on methodology to be used for developing and using PRO in oncology drug development programmes, and has emphasized the increased importance on patient input in clinical trials. In addition to QoL, many PRO questionnaires are used to assess complementary areas in oncology study, including: disease related symptoms (pain or fatigue), physical/social/emotional aspects, symptomatic adverse events, and satisfaction with the care provided.
Using eCOA for oncology trials can be particularly challenging because of the range of therapeutic approaches. Key components should be taken into consideration in your oncology study to ensure the success of eCOA implementation.
To download the infography, click here:
Last update : 21 February 2017

