Since 2005, Kayentis has been a trusted partner for pharma, biotechs, and CROs, capturing meaningful patient input. Our user-friendly eCOA technology and top-quality services not only ensure high-quality datasets and improve the experiences of patients and sites, but also streamline our clients’ journey.
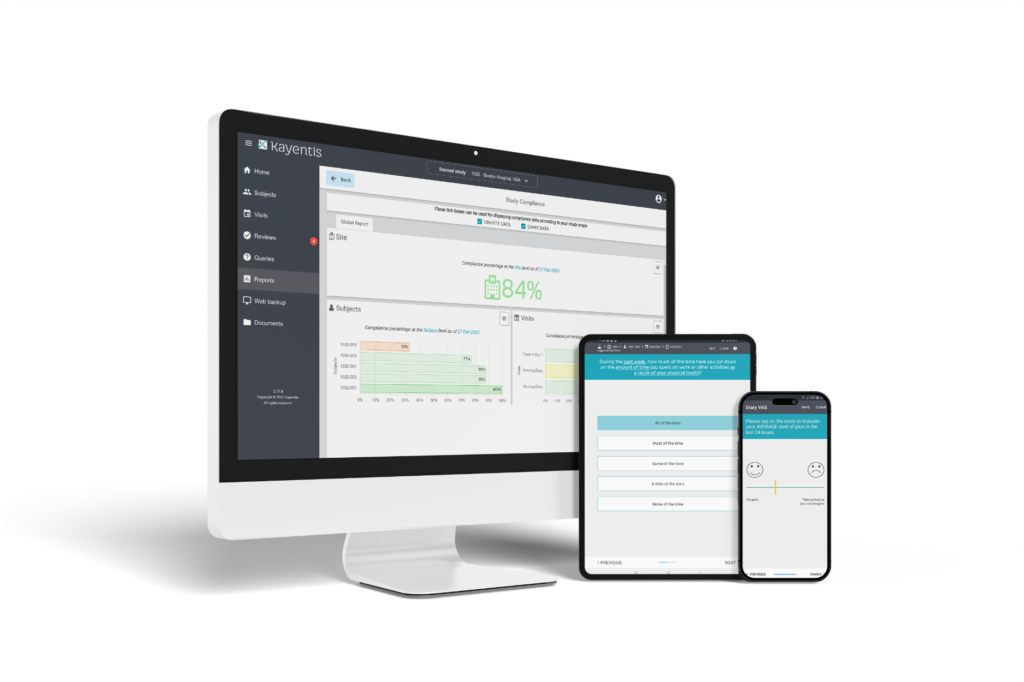


Early strategic engagement
Strong data management support
Proactive project management
Worldwide logistics
& 24/7 helpdesk support

At Kayentis, we aim to be a sustainable company in a sustainable world,
committed to improving human health.
We care
about the planet
We foster
well-being at work
We respect people
and encourage diversity
We value
our community