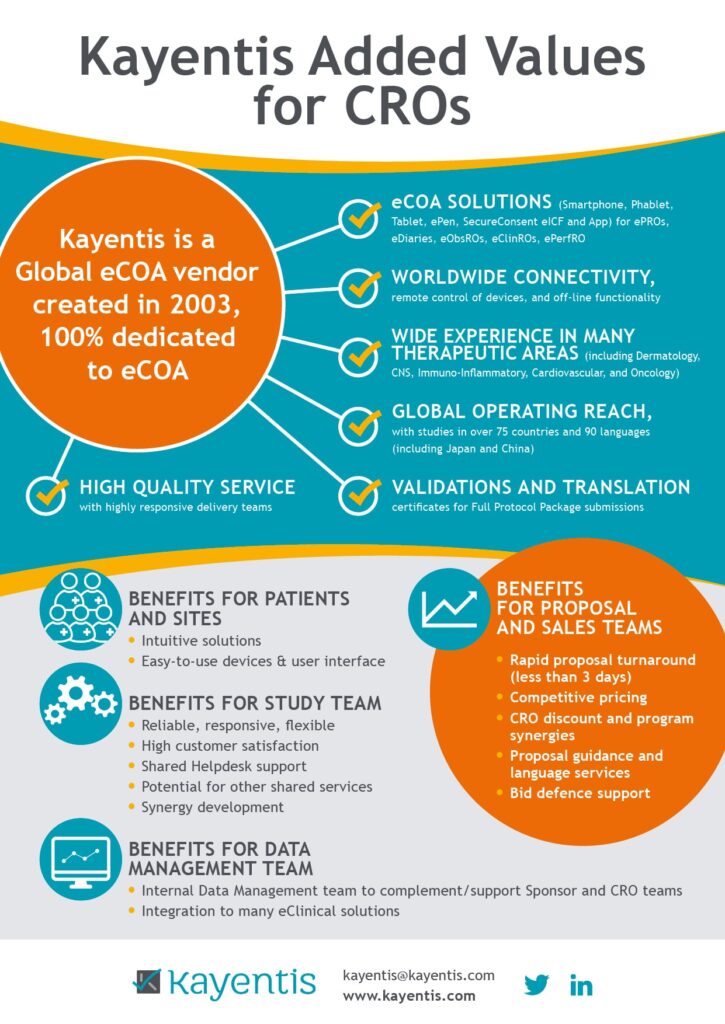
Kayentis is a global eCOA vendor created in 2003, 100% dedicated to eCOA. Here is an overall infography about Kayentis added values for CROs. Its experience is based on several criteria:
- worldwide connectivity
- wide experience in many therapeutics areas
- global operating reach
- high quality services
- and many others…
This infography also underlines the benefits for patients and sites, the benefits for study teams and those for proposal and sales teams, such as a rapid proposal turnaround, competitive pricing, CRO discount and program synergies…
To download “Kayentis added values for CROs”:
[tag url=”https://kayentis.com/wp-content/uploads/2018/01/kayentis_infography_added-values-for-cros.pdf” event=”Infography added values for CROs “]

Benefits for patients and sites
- Intuitive solutions
- Easy-to-use devices & user interface
Benefits for study team
- Reliable, responsive, flexible
- High customer satisfaction
- Shared Helpdesk supportPotential for other shared services
- Synergy development
Benefits for data management team
- Internal Data Management team to complement/support Sponsor and CRO teams
- Integration to many eClinical solutions
Benefits for proposal and sales teams
- Rapid proposal turnaround (less than 3 days)
- Competitive pricing
- CRO discount and program synergies
- Proposal guidance and language services
- Bid defence suppor
You can also read our article about the benefits & practicalities of aligning the objectives of an eCOA vendor with those of the study.
Guillaume JUGE, CEO – Kayentis