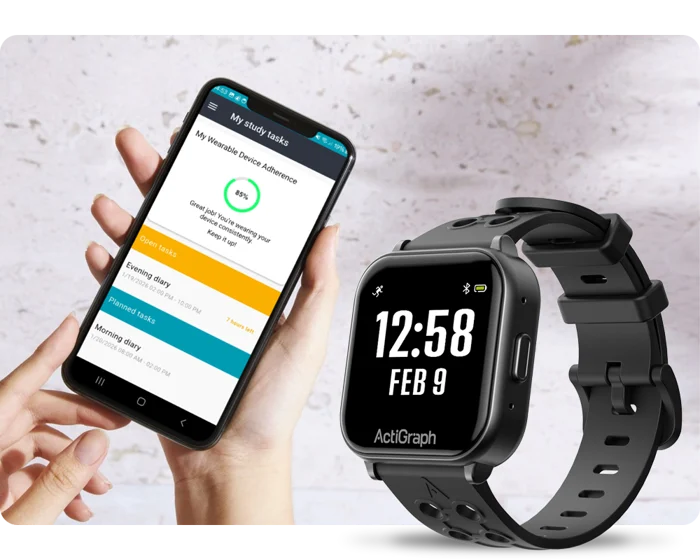
“Through the quality of the solutions, services, and databases that we provide to sponsors and CROs, we contribute to the reliability of clinical research and help to improve human health.“
In addition to our contribution to clinical research, we now actively contribute to reducing climate change, by controlling our carbon footprint.
Our global climate control engagement
Since 2016, we have been committed to reducing the carbon footprint of our internal activities:
- We launched a number of initiatives to control and reduce our carbon footprint: promotion of alternative options for employees commuting between home and office, control of work-related travel, monitoring of facilities including control of heat/air conditioning installations and IT equipment lifetime expansion.
- Every year we calculate the carbon footprint per employee of our company which allows us to closely monitor our carbon footprint trend. Knowing this, we can implement additional actions to improve our footprint from one year to the next.
- And each year, we make a donation to local health associations with the equivalent dollar amount of the CO2 amount of we cannot reduce.
In 2020, we have decided to go a step further, and are launching an additional initiative focused on our core business: our carbon-neutral program for clinical trials. We aim to reduce the carbon impact of each eCOA clinical trial and to offset the amount of CO2 that we cannot reduce.
Our initiatives to bolster carbon-neutral clinical trials
To reduce the carbon impact of the eCOA projects we conduct:
- We evaluate the carbon impact of each eCOA project we conduct together
- Depending on the protocol of the clinical trial study, we propose solutions to optimize carbon emissions for a project, that could include opting for:

- A webCOA backup strategy, using an online platform to directly enter patient data rather than stockpile spare devices on each site
- Replacing onsite training and face-to-face meetings with virtual ones
- Using refurbished devices – as the lifespan of a device is around four years, refurbishing is a feasible solution for both short-term studies and to replace a device during the course of a study
- We offset the amount of residual emissions of the eCOA project by making an equivalent monetary donation to the carbon solidarity action program at GoodPlanetFoundation.
This foundation aims to combat climate change by developing sustainable and economically viable alternatives to polluting activities, to the benefit of the most disadvantaged groups. This program combines actions to remove CO2 from the atmosphere (e.g. planting trees) or to avoid CO2 emissions (e.g. use of solar cookers instead of deforestation for cooking).
Contact us for more information about the program.
Jean-Michel Combe, Head of Strategic Marketing & CSR manager @Kayentis
Discover more: