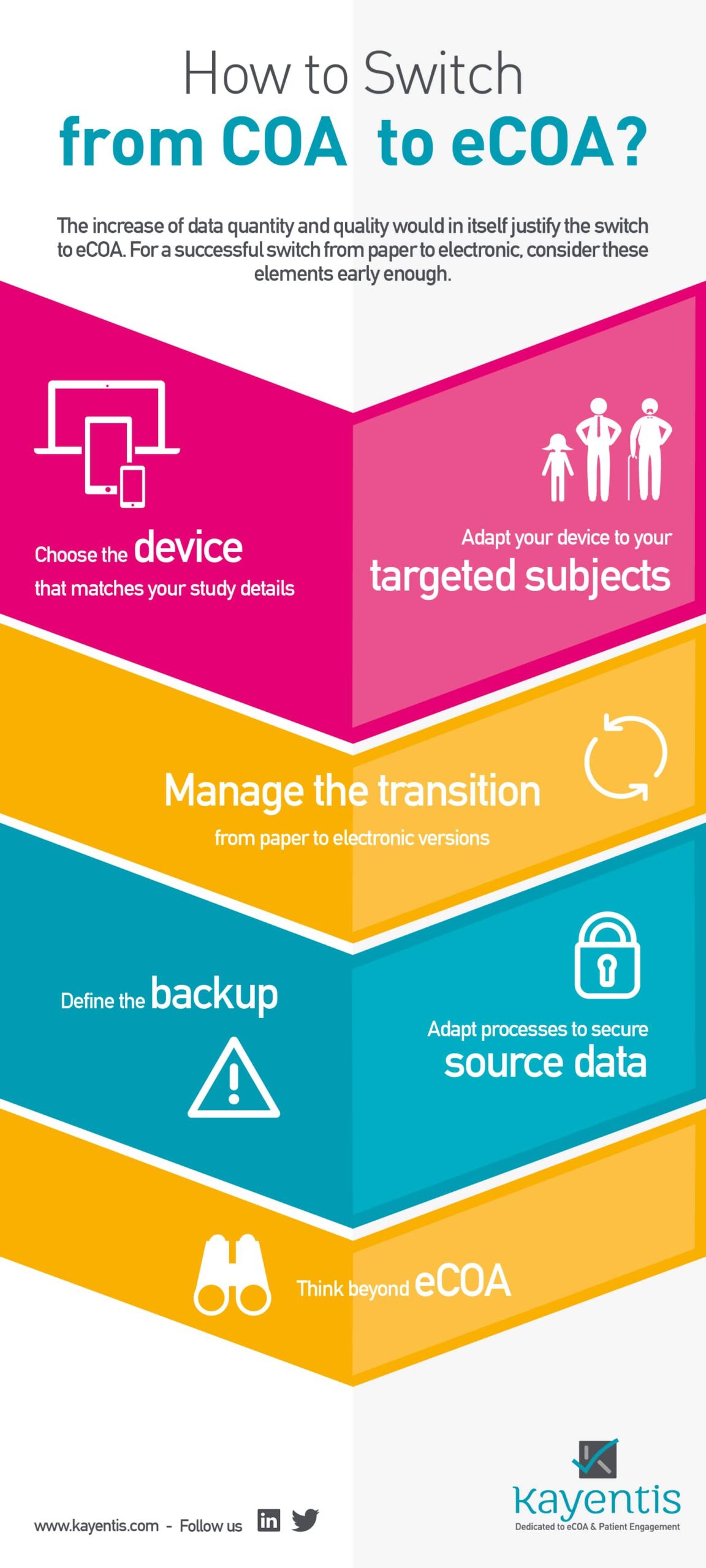
Infography COA to eCOA: in many cases this move from COA to eCOA generates cost cuts for the sponsor as well, by reducing drastically spendings on monitoring, data management, logistics and non-quality impacts; if the instruments chosen bear study endpoints, the increase of data quantity and quality would in itself justify the switch to eCOA.
For a successful switch from paper (COA) to electronic clinical studies (eCOA), consider 7 major aspects early enough. All of them are detailed in the infography below. Click here to know more about this switch.
Several vendors now propose eICF on top of eCOA on the same device for instance, generating savings for the sponsor while making it very simple for the sites and really informative for the subjects.
Moving forward towards eCOA is undoubtedly beneficial to the quality of the data and the trial, and implementation of eCOA solutions is expected to grow quickly in the next few years. In order to smoothly manage this transition, sponsors should strongly consider early work with translation companies and eCOA vendors to cope with the aforementioned challenges.
Guillaume JUGE, CEO – Kayentis