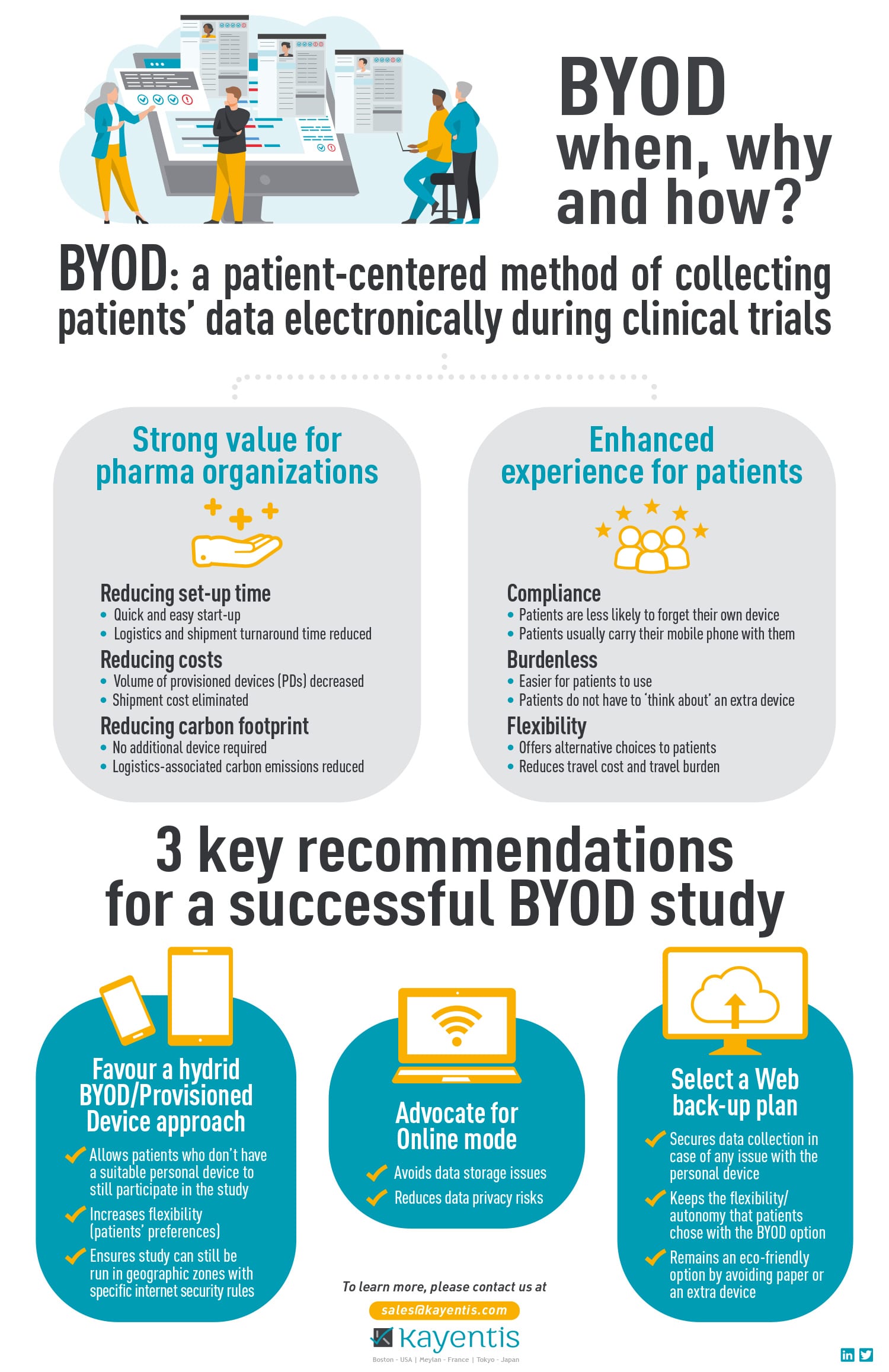
BYOD is a patient-centered method of collecting patients’ data electronically during clinical trials, providing strong value for pharma organizations and an enhanced experience for patients.
Discover the 3 key recommendations for a successful BYOD study below: