Actigraphy has been used in clinical trials for almost 20 years. Recently their inclusion in study protocols has been increasing, for several reasons: accelerometers have become inexpensive, the technology has been miniaturized, and the market is being flooded with a multitude of activity recording wearable devices. Thus, Kayentis’ team has been able to release its last case study about cardiovascular and heart failure.
In areas such as cardiovascular and metabolic research, study endpoints often include sleep and physical activity records. It is therefore important to have tools that can continuously monitor and record these data, including at home.
What is an actigraphy device?
- An actigraph, also referred to as an actometer or actimeter, is a wrist-worn activity monitor that is used to monitor movement and sleeping/waking patterns over an extended time period.
- The actigraphy device may be placed on the wrist, ankle, or trunk. In most studies, it is worn on the non-dominant wrist since the wrist detects more movement than the ankle or trunk. The actigraphy device includes a small accelerometer that monitors and records the occurrence and degree of motion. It can collect data continuously over an extended period of more than one week.
- Actigraphic data can be displayed and scored manually or downloaded to a computer for display and analysis by software and algorithms that give estimates of sleeping/waking patterns and circadian rhythm parameters. The collected data are translated into epochs (typically 30 seconds or 1 minute) of activity. Using validated algorithms, the epochs are scored as sleeping or waking. The device interprets the presence of movement as time awake, and absence of movement as sleep time.
Which therapeutic areas can be benefited by using this device?
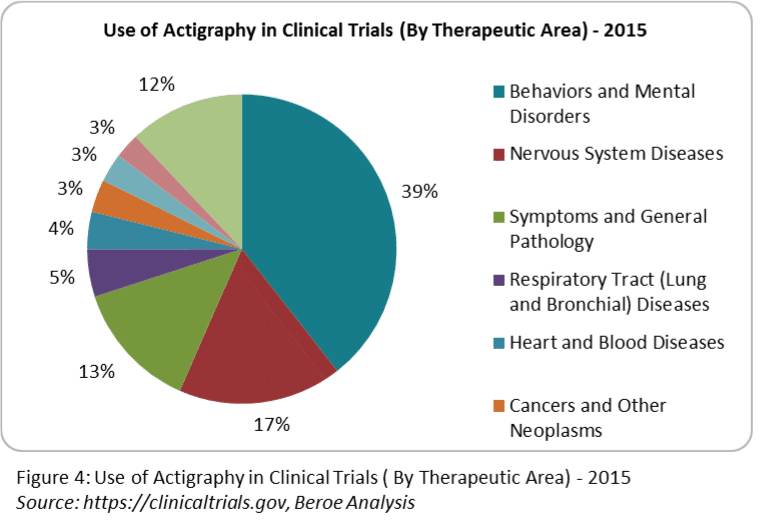
Actigraphs are widely utilized in behaviorial and mental disorders, neurological diseases such as Parkinson’s disease, Alzheimer’s disease, cerebral infarction, seasonal affective disorder, restless leg syndrome, vascular dementia and also sleep disorders related to abnormal activity symptoms. Actigraph has often been used for Primary and Secondary endpoint.
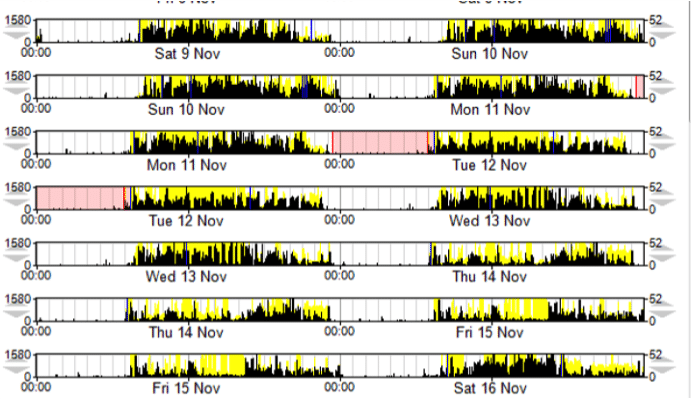
An example of actigraphy use in sleep disorders
- Diagnosis of a sleep disorder can require a clinical interview combined with polysomnography (PSG) (laboratory or home-based) and a sleep diary. Laboratory PSG is considered to be the “Gold standard” for the assessment of sleep but it is time consuming and expensive and not always feasible in clinical practice.
- The use of a sleep diary, which requires patients to complete their diary each day for maximum validity, is less simple than using questionnaires. Clinical interviews, which rely on patients’ memory, can introduce bias and their usefulness is limited in patients who are unable to self-report reliably (e.g., young children, patients with dementia).
- Alternatively, there are fewer restrictions on the population for whom wearable accelerometers such as actigraphs can be of use. These allow the long-term recording during daily activities and so are considered to be the best choice for the quantitative assessment of disease symptoms. Actigraphy also provides objective information about daily variability and sleep quality. Actigraphy appears to provide a valid estimate of total sleep time, sleep percentage, and periods of waking after sleep onset.
-
Furthermore, it provides useful information for the measurement of sleep habits in terms of the evaluation of insomnia and circadian rhythm sleep disorders, and is a means of estimating Total Sleep Time (TST) in the recording of sleep-related breathing disorders.
The ease of use and good compliance of actigraphy allow long-term outcome studies and repeat tests to be conducted, which is very costly or not possible using other technologies. An eCOA provider such as Kayentis has the knowledge and capacity to collect, process, and store activity data.
Not all actigraphy devices are the same, and it is critically important that reliable, validated systems are in place for drug development evaluations.
// Click here to discover our case study about cardiovascular, heart failure and actigraphy //
Sophie Althuser, Project Manager – Kayentis
Sources
www.provider.ghc.org
www.appliedclinicaltrialsonline.com
Chest. 2011 Jun; 139(6): 1514–1527. Wrist Actigraphy, Jennifer L. Martin, and al.
McCarthy M, Muehlhausen, W ICON PLC, South County Business Park, Leopardstown, Dublin 18, Ireland








