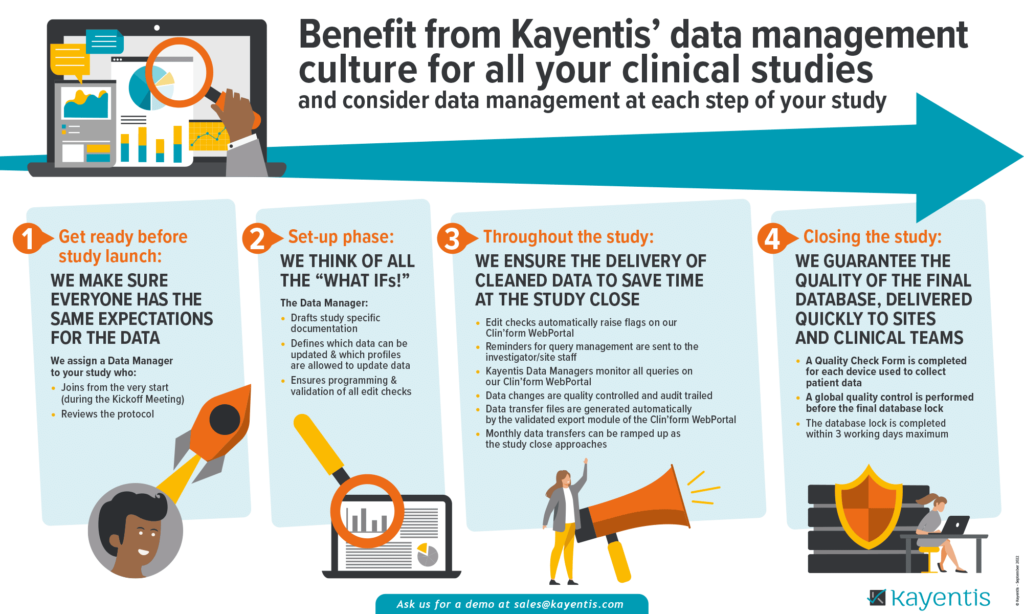
At Kayentis, we:
- Make sure everyone has the same expectations for the data
- Think of all the “what ifs!”
- Ensure the delivery of cleaned data to save time at the study close
- Guarantee the quality of the final database, delivered quickly to sites and clinical teams