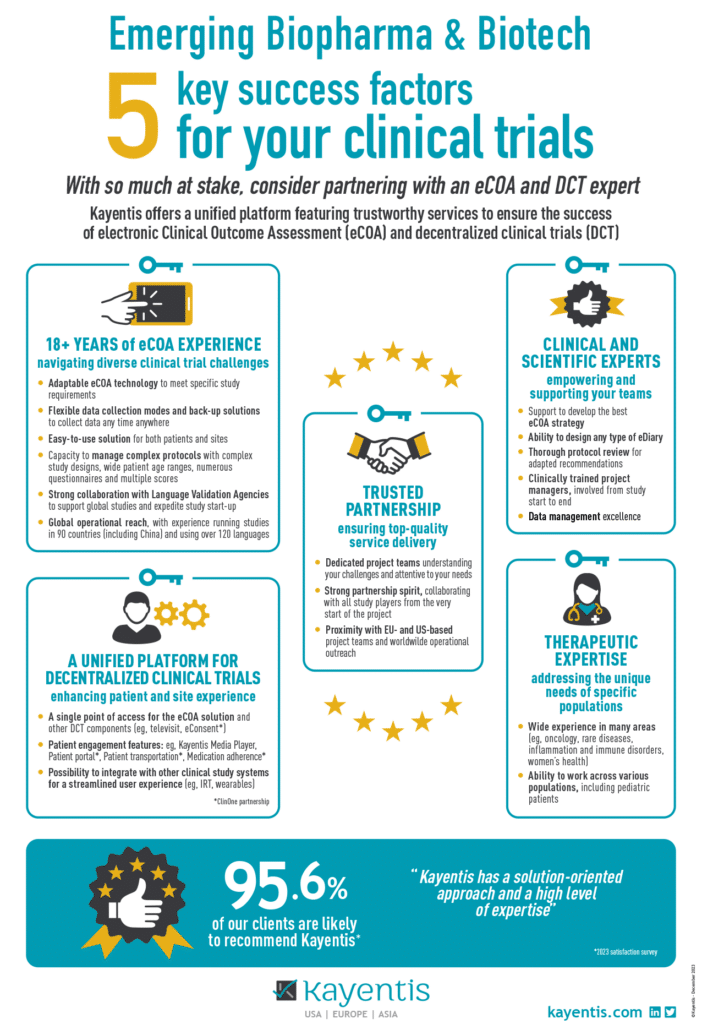
With so much at stake, consider partnering with an eCOA and DCT expert!
Kayentis offers a unified platform featuring trustworthy services to ensure the success of electronic Clinical Outcome Assessment (eCOA) and decentralized clinical trials (DCT):
- 18+ years’ eCOA experience navigating diverse clinical trial challenges
- A unified platform for decentralized clinical trials enhancing patient and site experience
- Trusted partnership ensuring top-quality service delivery
- Clinical and scientific experts empowering and supporting your teams
- Therapeutic expertise addressing the unique needs of specific populations