With the increasing importance of direct patient input into clinical trials, most protocols combine ePRO and eDiary questionnaires for use on-site and at home, respectively. This allows the evaluation of complementary areas such as disease-related symptoms, physical/social/emotional aspects, symptomatic adverse events, treatment compliance, as well as aspects such as patient satisfaction, the value of which has grown increasingly in recent years.
Combining ePRO and eDiary solutions on one device presents several advantages, including increased simplicity and overall time- and cost- savings, while at the same time making the clinical trial experience more enjoyable for patients, study sites and monitors. What are these benefits?
5 reasons to choose a single device for both home and on-site use


A mid-size device can be selected
There are advantages in using multiple devices in some studies, where tablets are used on-site but the portability of a smartphone for data collection at home can be a real advantage, e.g. for eDiary completion. However, for most clinical trials, the larger screen size and comfort of a tablet are often highly appreciated, bringing several benefits including ergonomic advantages, better display of assessment scales, the possibility of data entry in landscape mode, and large buttons.
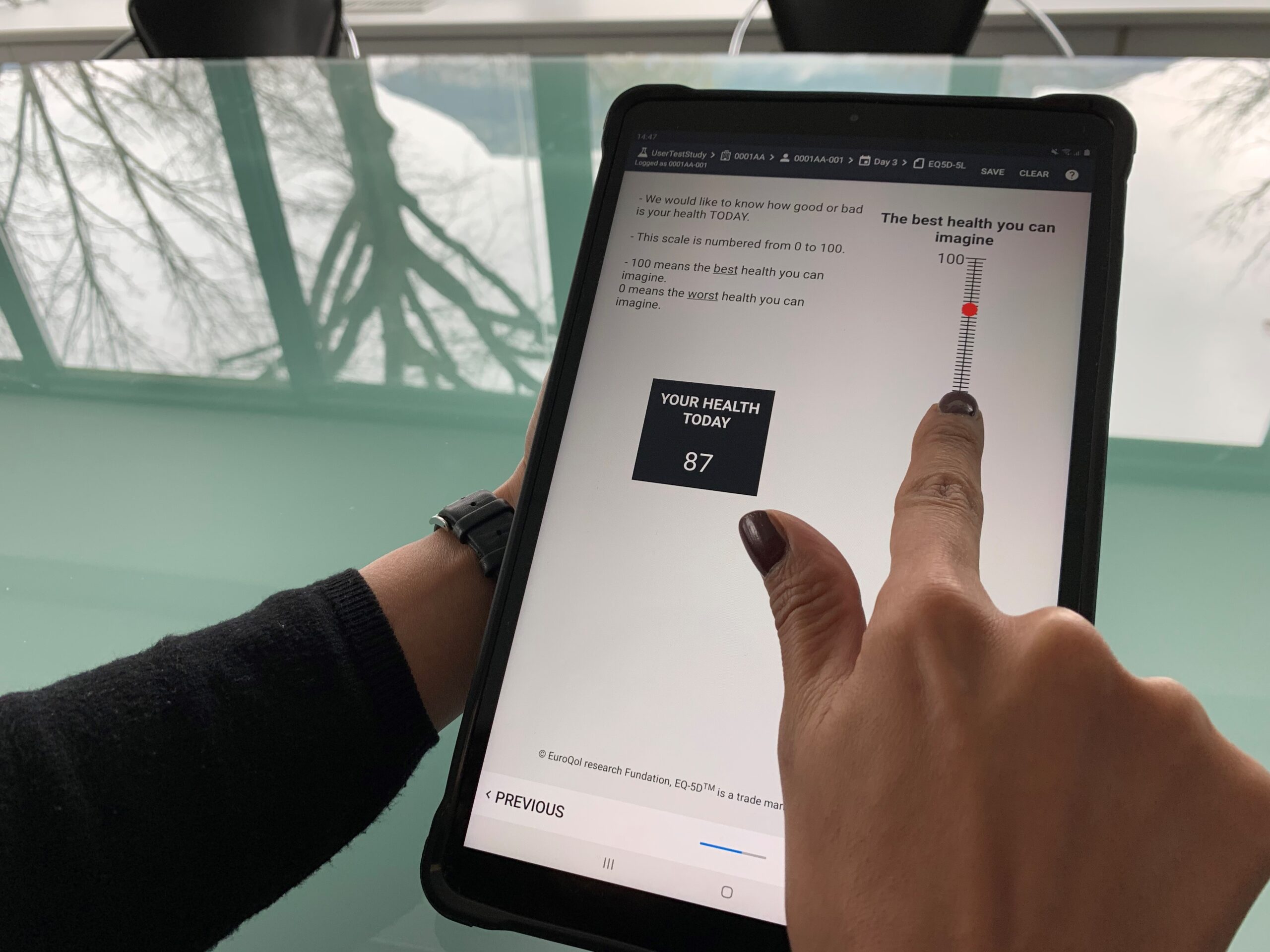
The relatively small size of Kayentis’s tablet (7’’or 8’’) makes it the ideal user-friendly handheld device for both ePRO data collection on-site and home-based eDiaries: it is light and portable whilst still offering larger displays than smartphones, which may be of value in certain contexts.

A single device reconciles specific needs
Small tablets can reconcile the needs of certain pathologies and populations.
- A benefit for specific populations:
Using multiple devices for studies enrolling elderly patients is unlikely to be patient-centric or adapted to the patients’ needs. The generous screen size, larger buttons, more legible font sizes and high resolution of a tablet for both home and on-site use are likely to be required.
When additional features are integrated – e.g. for pediatric populations – to make the questionnaire more appealing, the landscape mode or the use of images may well be required for specific features and so will require the same device to be used at home and during the site visit.
- A benefit for specific therapeutic areas:
There is an increasing importance of direct patient input into clinical trials, and some therapeutic areas (such as oncology, immune inflammation and rare diseases) require adapted display of assessment scales. For example, a larger screen may be required to provide an optimal presentation of the rating scales and their instructions. A generous screen size is also particularly important in the completion of Visual Analog Scales (VAS) or when images are used to support the completion of specific questionnaires or eDiaries at home.
- A benefit for specific protocols:
When data are to be completed both on-site and at home, users may prefer to combine ePRO and eDiary data collection on the same device rather than using two separate devices. Also, when the eDiary does not require more than one daily completion, patients may complete their diary at home in the morning or in the evening on a tablet and so do not necessarily need to carry a device around all day. Kayentis’ small tablet, having both supervised and unsupervised modes, can be used on-site as well as at home.

A better clinical trial’s experience for the patient
- Simplicity: it is simpler and easier for several users to handle one rather than two devices, and to have all the necessary information relevant for the study on a single device – More handy, user-friendly approach.
- Reduced training burden: focusing on one device reduces the training duration and recurrence – Reduced burden, better adherence.
- Making clinical trials an enjoyable experience: the handheld tablet device has high quality touch screens and is very intuitive, which is key to increasing data quality while ensuring patient compliance – Increased comfort, stronger patient engagement.

Main advantages for the site
- Flexibility: when used onsite, the investigator initiates the visit and invites the patient to complete the ePRO. At the end of the visit, the investigator returns the tablet to the patient – Simple, easy, streamlined process.
- Training efficiency: training for both for the site’s staff and that provided by the site for the patients is facilitated by using a single device – Reduced burden, increased efficiency.
- Simple review process: the Kayentis interface for reviewing patient questionnaires on the tablet device ensures higher data quality and helps to increase site engagement – Simple process, clear overview.
- Reduced monitoring burden: eCOA logistics, device reuse, and patient reallocation are simplified. The supply logistics and inventory management, with preparation, shipment, and logistics of the devices, are simplified. Hence, the site set-up workload is reduced, which can be crucial for the site’s resources – Easier logistics, optimization of resources.

Added value for the sponsor and/or the CRO
- Simplified monitoring: eCOA logistics, support, and training are simplified by being included on a single device instead of multiple devices, which has a positive impact on the associated support and monitoring – Stronger support, optimized resources.
- Competitiveness: significant time- and cost-savings on trial set-up and management due to fewer devices and more streamlined logistics – Time savings, cost reduction.
- Better compliance and data quality: by improving patient compliance in ePRO and eDiary completion, and the commitment and productivity of trial site staff as well as increasing the efficiency of data collection, the clinical trial’s chance of success is increased – Improved patient and site engagement, better data quality, higher chance of success.
Combining ePRO and eDiary solutions in a single device adds value for a range of clinical trials stakeholders, but, as is often the case, there’s no golden rule. The study objectives and design, as well as a consideration of the patient population and the specifics of the disease remain necessary to ensure that the right eCOA solution and device strategy are selected
Frédérique MARION, Director Business Development @Kayentis
Discover more:













