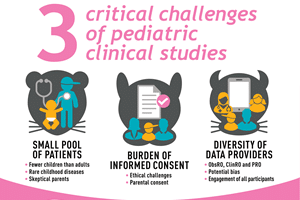
Approximately one in every five pediatric clinical study fail1,2 . A 2016 study shows that over a three-year period (2008 -2011), more than 77,500 children participated in pediatric clinical studies that contributed little or nothing to advance treatments for their illnesses because the research disappeared from scientific view3: what challenges cause these failures and how can they be overcome?
- Learn more about these challenges and the tips to overcome them here.

To download “Overcoming complexities in pediatric clinical studies”:
[tag url=”https://kayentis.com/wp-content/uploads/2020/04/kayentis-overcoming-complexities-in-pediatric-clinical-studies.pdf”]
Discover more:
References
[1] https://pediatrics.aappublications.org/content/138/3/e20160223.long
[2] https://www.ncbi.nlm.nih.gov/pubmed/17329988
[3] https://www.scientificamerican.com/article/many-pediatric-studies-are-a-waste-of-time/