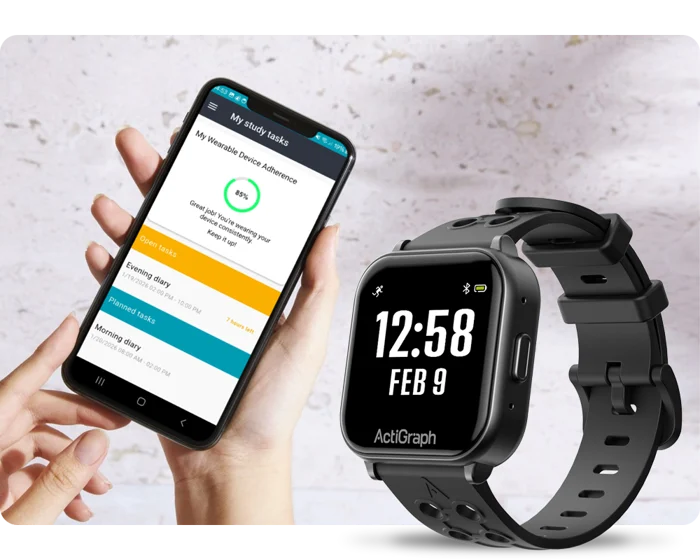
More than simply unifying wearable and patient-reported data in one app, integrated solution directly tackles major challenge in wearable enabled clinical trials: wearable device adherence. By embedding real time compliance monitoring and guidance within the eCOA app, it eliminates fragmented tools and reduces inconsistent device use, boosting patient engagement, data quality and site efficiency.
Grenoble, France , and Pensacola (FL), USA, January 28, 2026 – Kayentis, a global provider of electronic Clinical Outcome Assessment (eCOA) and Decentralized Clinical Trial (DCT) solutions, and Ametris (formerly ActiGraph), a global digital health solutions provider transforming real-world patient data into validated clinical evidence, today announce a strategic partnership to deliver an integrated eCOA and wearable technology solution transforming wearable device adherence in clinical trials.
By integrating continuous, real-world wearable technology monitoring and management directly into the patient’s eCOA app, it enables them to complete eCOA questionnaires and tasks in this combined solution, while providing essential support, improving wearable compliance and reducing data variability.
Wearable technology embedded features within Kayentis’ eCOA app designed to support patient’s device adherence include:
- Real‑time feedback on whether they’ve worn their device sufficiently (i.e., smart watch)
- Helpful reminders on how to improve compliance with their wearable device
- Automatic syncing of their wearable device
Kayentis and Ametris’ partnership addresses one of the most pressing challenges: ensuring sustained patient device wear adherence in today’s increasingly digital and complex clinical trials, particularly in oncology, cardiology and neurology indications.
For sites, this integrated approach simplifies enrollment and device management by reducing the number of systems and workflows staff must oversee. Sponsors, in turn, benefit from more engaged participants, improved data completeness and the potential for operational efficiencies related to devices, connectivity and support.

“We are thrilled with this alliance with Ametris in rolling out a value-added integrated eCOA and wearable technology solution that will transform wearable adherence in clinical trials. It marks a significant step forward in the management of complex clinical trials, often marred by data frequently collected in separate systems and only reconciled retrospectively,” said Guillaume JUGE, CEO of Kayentis. “By joining forces, we are expanding our capabilities while making life far easier for patients, sites and sponsors.”
Integrated solution addresses challenge in wearable device adherence
In clinical research, wearable adoption continues to accelerate, particularly in oncology, cardiology and neurology trials, where wearable devices are increasingly used to capture objective measures of sleep, circadian rhythms, activity, functional capacity and vital signs. However, poor wearable device adherence in clinical trials remains one of the major sources of data variance, operational burden and cost.
Unlike existing solutions that merely merge data for sponsors, the Kayentis–Ametris integrated solution tackles the challenge of long-term wearable adherence head-on by embedding the compliance monitoring of Ametris’ ActiGraph LEAP multi-sensor wearable into Kayentis’ eCOA application, Clin’form App. Mobile alerts notify patients when their device wear time is insufficient, prompting corrective action, thereby improving compliance and the reliability of collected data.

“This partnership represents a meaningful step forward in how digital health technologies are delivered in clinical trials,” said Jeremy Wyatt, CEO at Ametris. “By deeply integrating our best-in-class wearable DHT platform with Kayentis’ proven eCOA capabilities, we’re addressing two of the most persistent challenges in studies today – participant adherence and site burden. Together, we’re enabling a more seamless experience for patients and sites while ensuring sponsors can rely on high-quality, continuous real-world data to support confident decision-making.”
The Kayentis-Ametris collaboration brings together two market-leading offerings: Kayentis’ eCOA platform, which caters to over 200 indications in 20 therapeutic areas and has been simplifying and improving clinical trial data collection since 2005, with Ametris’ best-in-class digital health technology (DHT) platform, combining validated clinical sensors, state-of-the-art algorithms, remote data capture solutions and seamless study operations, to power continuous, patient-focused data collection across a wide variety of therapeutic areas.
The partnership aims to evolve, with future enhancements planned to further streamline device management and trial operations.
About Ametris
Founded in 2004, Ametris (formerly ActiGraph) is a global digital health solutions provider transforming real-world patient data into validated clinical evidence. Our end-to-end platform combines advanced wearables, regulatory-aligned analytics and expert scientific support to simplify every phase of a clinical trial – from study design to submission. Backed by two decades of experience and a collaborative approach, Ametris empowers research teams to run smarter trials, reduce patient burden and accelerate therapeutic innovation.
www.ametris.com
About Kayentis
Kayentis is a global leader in electronic Clinical Outcome Assessment (eCOA) and Decentralized Clinical Trial solutions. Since 2005, Kayentis has supported pharma, biotechs and CROs in streamlining data collection and enhancing the quality of clinical trials. With expertise spanning all phases (I–IV) and more than 200 indications across over 20 therapeutic areas, Kayentis delivers a comprehensive suite of services designed for science-driven and patient-centric research. Operating across the US, Europe and Asia, Kayentis is committed to innovation and excellence, continually adapting its solutions to meet the growing complexity of clinical trials and to improve outcomes for sponsors, sites and patients.
kayentis.com
Media & analyst contact
Andrew Lloyd & Associates
Carol Leslie / Juliette Schmitt
carol@ala.associates / juliette@ala.associates
UK: +44 1273 952 481
US: +1 203 724 5950