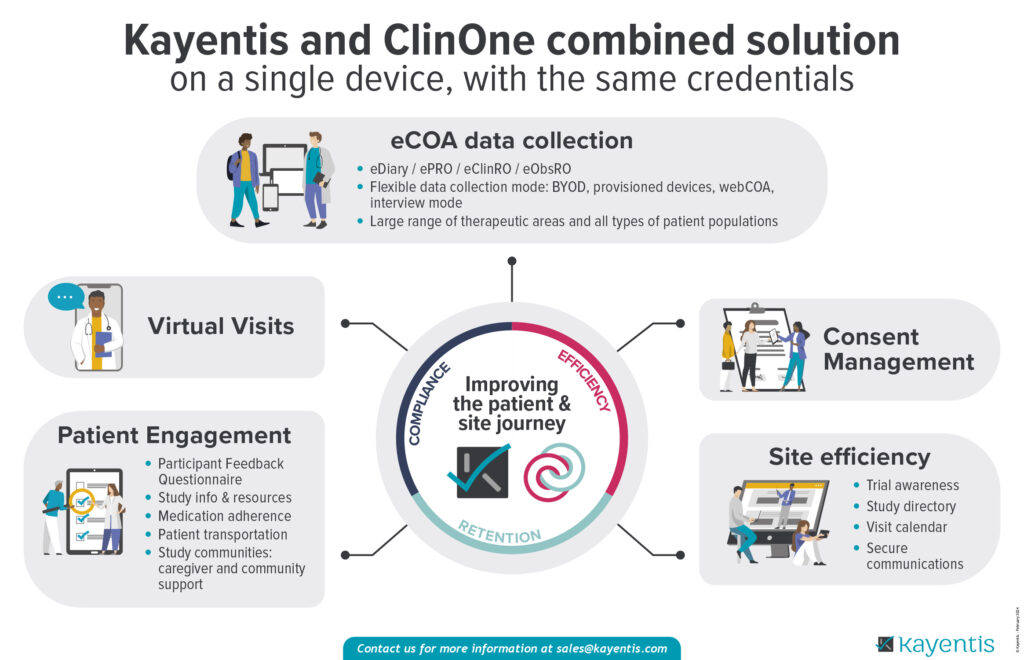
Improve your eCOA data collection and clinical trial efficiency thanks to Kayentis and ClinOne combined solution!
Ease the patient and site journey with a unique access to:
- eCOA data collection
- Consent Management
- Virtual Visits
- Patient Engagement features
- Site efficiency features