A multi-platform eCOA solution to address the clinical trial needs of the future
Over the last 2 years, Kayentis has been redesigning its software solution for the management of clinical trials – Clin’form – to anticipate future developments in clinical trials.
This new design includes a multi-platform solution for mobile use to integrate and automate the collection of data from medical devices, to anticipate the introduction of ‘Bring Your Own Device’ (BYOD) approaches to the most common operating systems (Android and IOS).
In brief, this is the development of a platform for the clinical trials of tomorrow!
What is Clin’form?
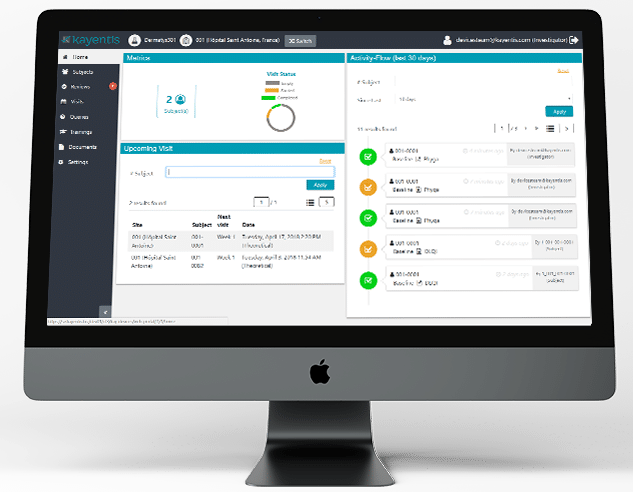
Clin’form is a software Suite designed and developed by Kayentis for the collection and analysis of electronic Clinical Outcome Assessment (eCOA) data in clinical trials.
It’s a multi-platform solution that allows data to be collected irrespective of the device used for data capture (web, mobile, tablet, BYOD, medical device), whether at the study site or at home.
From the web interface, various stakeholders – including sponsors, investigators, monitors, and data managers – can observe, analyse, or interact to ensure the good conduct of the clinical trial.
After 14 years of experience in eCOA, we are happy to launch this new major update!
How did you develop this new solution, Clin’form3?
- We wanted to develop cutting-edge technology
The idea was to develop the platform of the next 10 years: a state-of-the art technology with an evolutionary architecture and graphic design reflective of modern guidelines, including adherence to recent ‘Material Design’ guidelines, to prioritize the user’s experience. We developed a Human-Machine interface that is intuitive and ergonomic.
- We chose robust technology
For example, by using the Google Angular5 we chose the most recent frameworks to take into account future market changes. We selected the most commonly used frameworks, tested by millions of people. They provide a solid base that secures future developments.
- We were committed to remain within the budget constraints of clinical trials
We chose Android to address the essentials of the market while managing the demanding budgets of clinical trials.
The IOS version will be available very soon to allow for full BYOD capability.
- We worked to the principle of continuous, ‘flexible’ process improvement
Since 2016, our development teams have had a ‘flexible’ approach to working. In practice, this means that we work with an approach of continuous improvement. We put in place automatic tests with performance indicators to ensure the quality of the solution being developed. Every night the systems are tested, and improved the following day. Each functional team is fully integrated into a collaborative way of working – project managers, data managers, and commercial functions. In this way, we reverse development practices by thinking of client needs before thinking about the product. This allows a much quicker response to client requests and the ability to adapt and continue to anticipate the market expectations to maintain a high level of reactivity, quality, and performance.
What benefits can we expect from Clin’form3?
The keywords are quality, performance, and adaptability!
Clin’form3 is much more flexible and powerful than its predecessor (Clin’form2) and offers lots of flexibility in the set-up and conduct of clinical trials.

Simplified clinical trial set-up, irrespective of the complexity of the trial protocol
- A set-up phase that is always faster.
- An even more flexible layout to meet the needs of questionnaire authors and to provide an unprecedented user experience in terms of ergonomics, design, and speed.
- The management of multiple access, to better address ObsRo clinical trials that have a third party involved in data collection.
- Simpler management of complex protocols: e.g. oncology protocols of unlimited duration, protocols that require a change of questionnaire during the trial conduct. Clin’form3 can adapt to any protocol requirements.
- More streamlined management of large volumes of data thanks to better performance.

A major step forward for monitoring!
We are putting in place indicators and allowing risk-based monitoring, the prevention of deviations, and the installation of alert systems to improve the management of a clinical trial and to improve visibility and reactivity.

A real advantage in clinical trial compliance
As well as the improvement in the experience of the user, the use of indicators and automated handling of ‘missing data’ means that Clin’form3 provides instant compliance indicators.
…and of course we keep the best bits of our previous version:
- Off-line functionality when there is no connection available.
- Rapid and reliable set-up.
- An application that is still simple to use.
- And the continued delivery of kits that are ready-to-use.
When will this new version be available?
Between now until beginning of 2019, all our new trials will be set up with Clin’form3. Clin’form3 is already available for all trials using supervised instruments and will be available in September 2018 for trials conducted at home (eDiaries): the first of these trials are already in a test-phase.
Clin’form3 falls completely within our objective of accelerating innovation in clinical trials and addressing aspects of clinical trials of the future: to address complex demands, improve quality and reactivity in the set-up and conduct of clinical trials, to develop risk-based monitoring, improve patient engagement, and improve the user experience.
For more information on Clin’form3, contact us to request a demonstration.
Sergio Caldarelli, Data Manager
Louis Chapu, Project Manager
Jean-Michel Combe, Strategic Marketing Manager
Vincent Ganneau, Teach lead developer
Romain Mansuy, R&D director deputy
Arnaud Michelet, R&D director deputy