EMERGING BIOPHARMA – BIOTECH
the now and the future of clinical research
Emerging BioPharmas (EBPs) are changing the landscape of drug development by developing a large portion of all innovative new medicines.
Here at Kayentis, we understand the unique challenges that EBPs face: as they are active in only a limited number of project developments, with limited resources, the stakes for success are extremely high.
- Our solutions support EBPs to develop innovative therapies and respond to unmet medical needs
- Our customized solutions address the complexity of each clinical protocolOur flat organization and flexibility ensure a trustful and efficient collaboration
Your trial, our expertise
“Kayentis is looking forward to partnering with each of our new clients on their journey to successfully bringing their products to market or having their assets acquired. Kayentis’ therapeutic expertise and experience conducting electronic data gathering for patients in clinical trials at global levels bring proven, quality outcomes. We have the reliability and flexibility EBPs require.”
EXPERIENCE & EXPERTISE
- Supporting more than 15 EBPs and Biotech companies in recent years
- Key therapeutic expertise in promising therapies: rare disease, oncology, inflammation/immune disorders...
SERVICES & FLEXIBLE TEAMS
- Strong customer service support
- Reactivity & flexibility
- Proximity with regional teams focused on your goals
- Scientific support
CONFIGURABLE ECOA SOLUTION
- Our capabilities and ability to customize solutions
- Configurable products to fit each study requirement
Your challenges, our experience
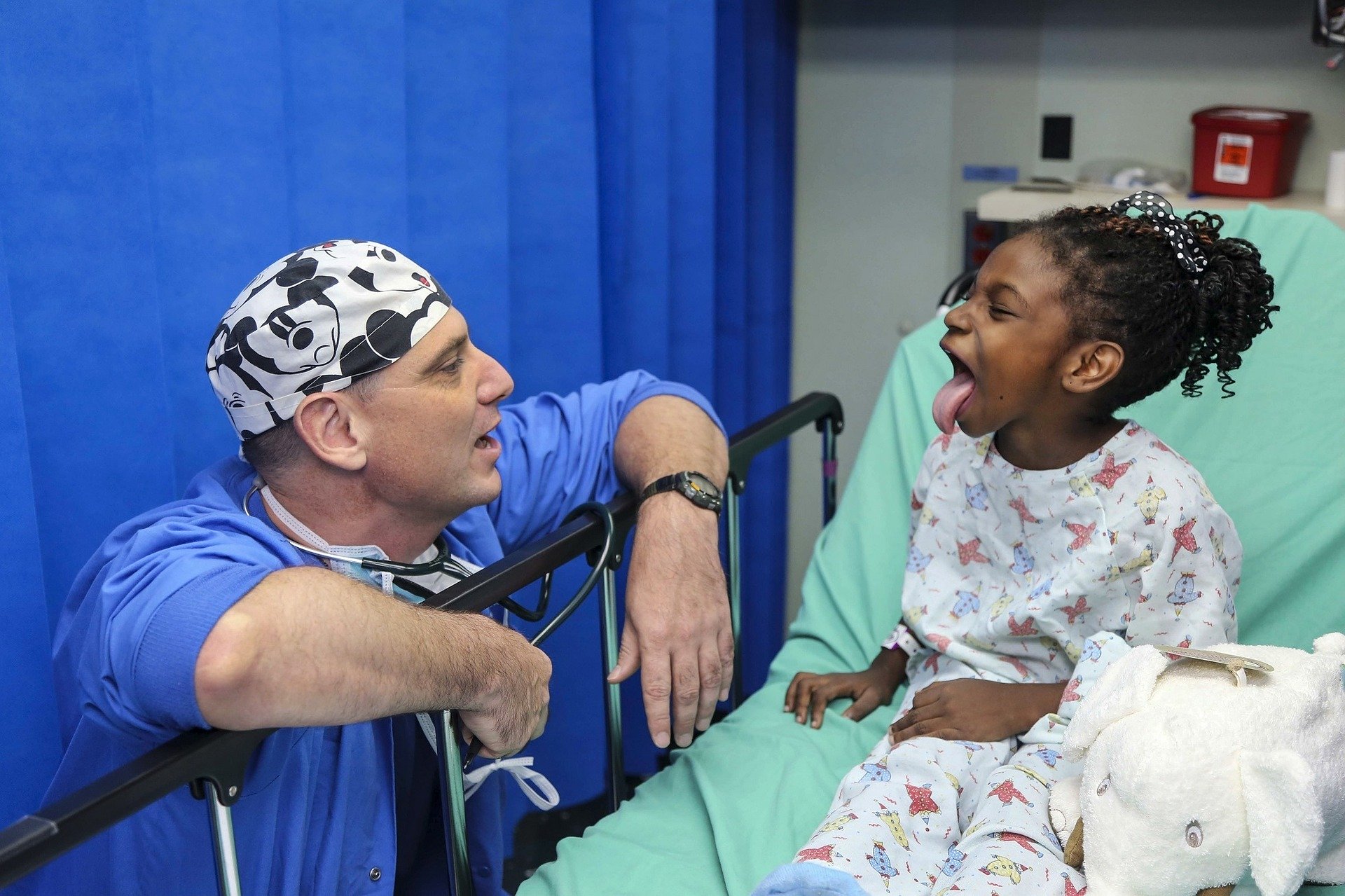
Overcoming the Complexities of Pediatric Clinical Trials
Although children make up 27% of the world’s population, at least 50% of all drug products may still lack labeling with pediatric information and approximately one in every five pediatric trials fail.
What challenges cause these failures and how can they be overcome?

Misconceptions on Barriers to eCOA adoption
Electronic Clinical Outcome Assessment (eCOA) solutions are increasingly preferred to paper questionnaires for endpoint data collection in clinical and epidemiological studies. However, challenging barriers hinder the widespread use of eCOA.
What are these barriers and are they wrongly perceived as being insurmountable?

//White paper// eCOA vs COA: pros and cons
The wide use of eCOAs in clinical trials has largely confirmed the theoretical advantages of electronic over paper questionnaires, including improved data quality and better confidence in these data.
Learn more about the pros and cons regarding eCOA vs COA solutions.


