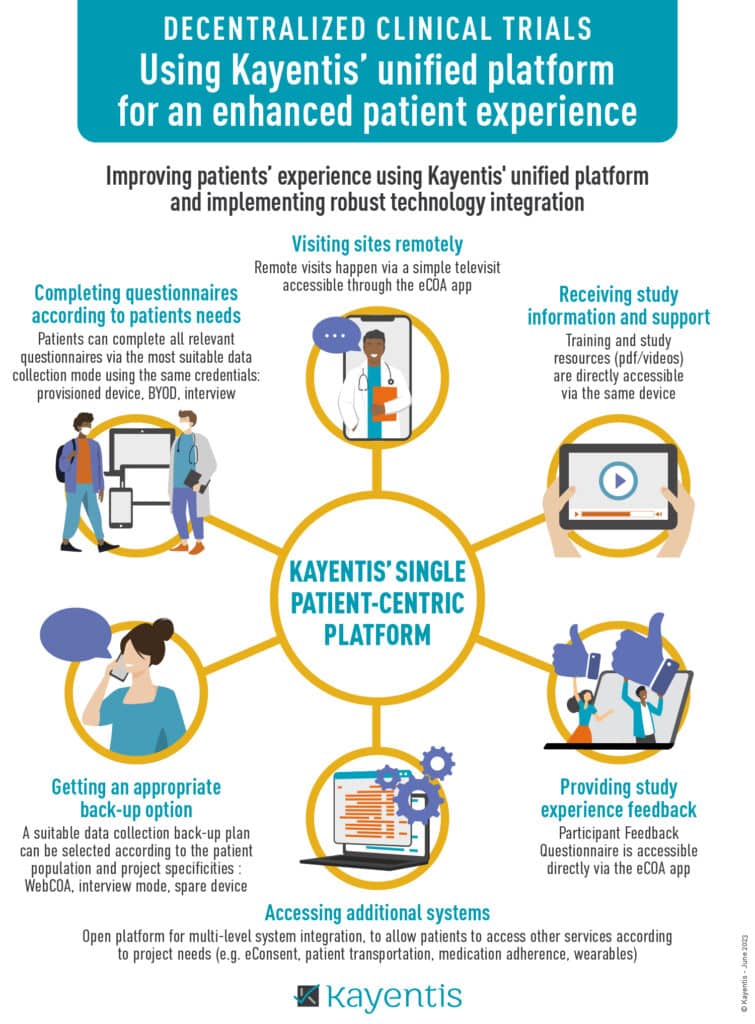
Improving patients’ experience using Kayentis unified platform and implementing robust technology integration.
- Visiting sites remotely
- Receiving study information and support
- Providing study experience feedback
- Accessing additional systems
- Getting an appropriate back-up option
- Completing questionnaires according to patients needs