Article
Empowering patients through education – A structured approach to clinical trial engagement
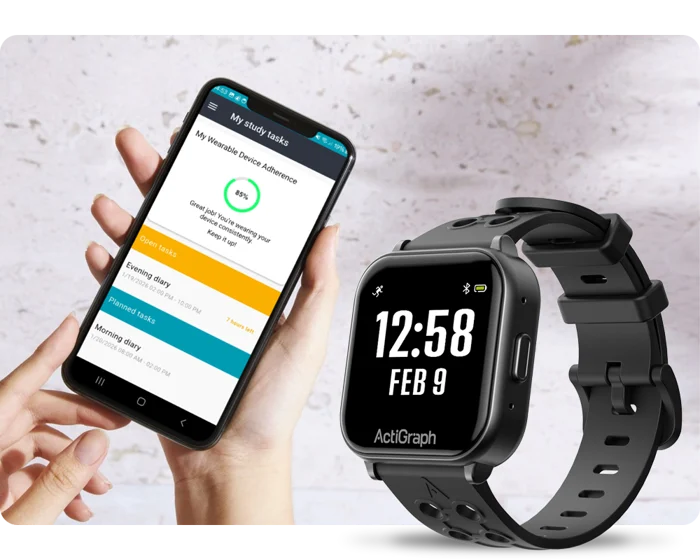
In the evolving landscape of clinical research, patient engagement has emerged as a cornerstone of successful clinical trials. In addition to improving recruitment and retention of patients, empowerment through education...