White Paper
ePRO in early phase oncology trials: A revolution on the horizon
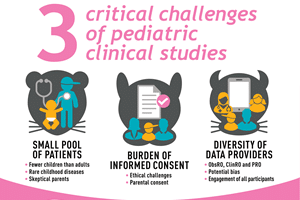
While the integration of Patient-Reported Outcomes (PROs) into phase IIb and III clinical trials has become increasingly widespread in the 21st century, their inclusion in early-phase studies, namely Phase I...