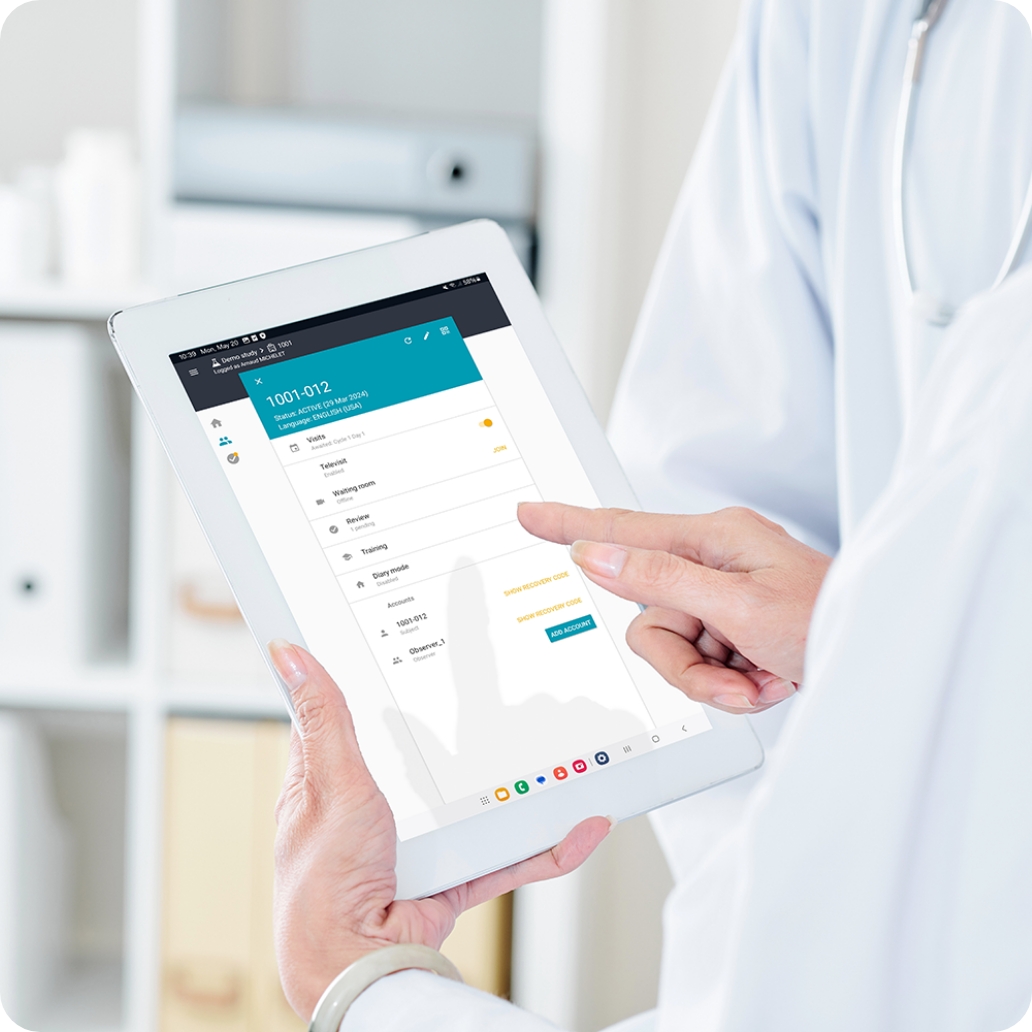
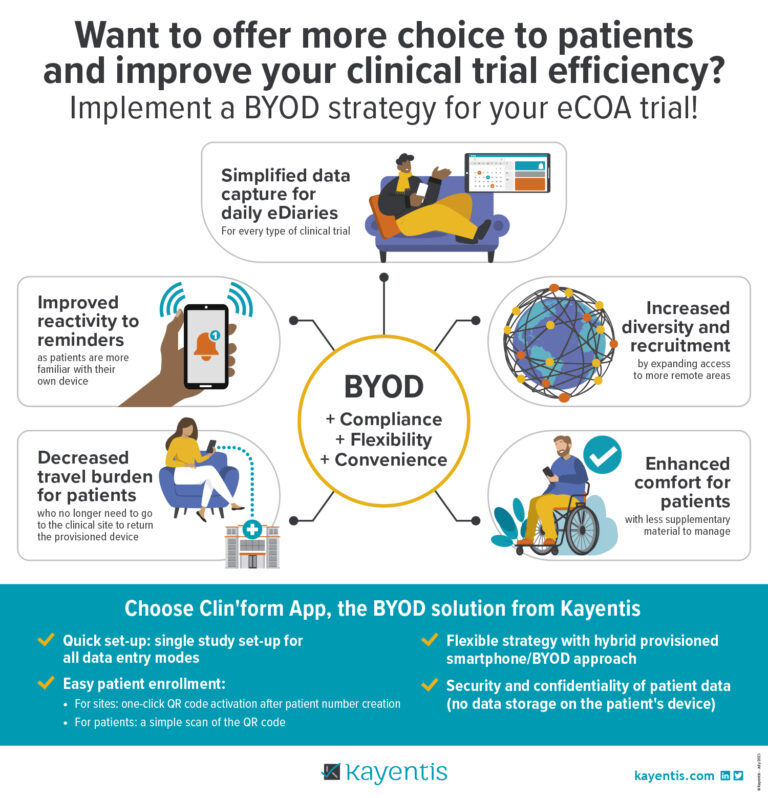
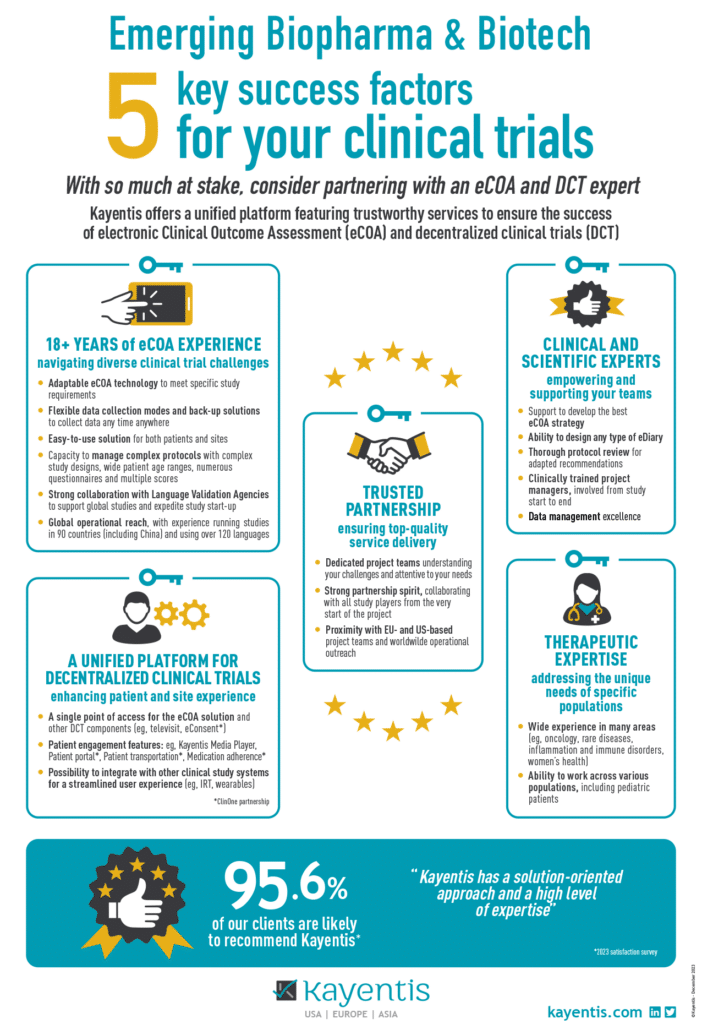
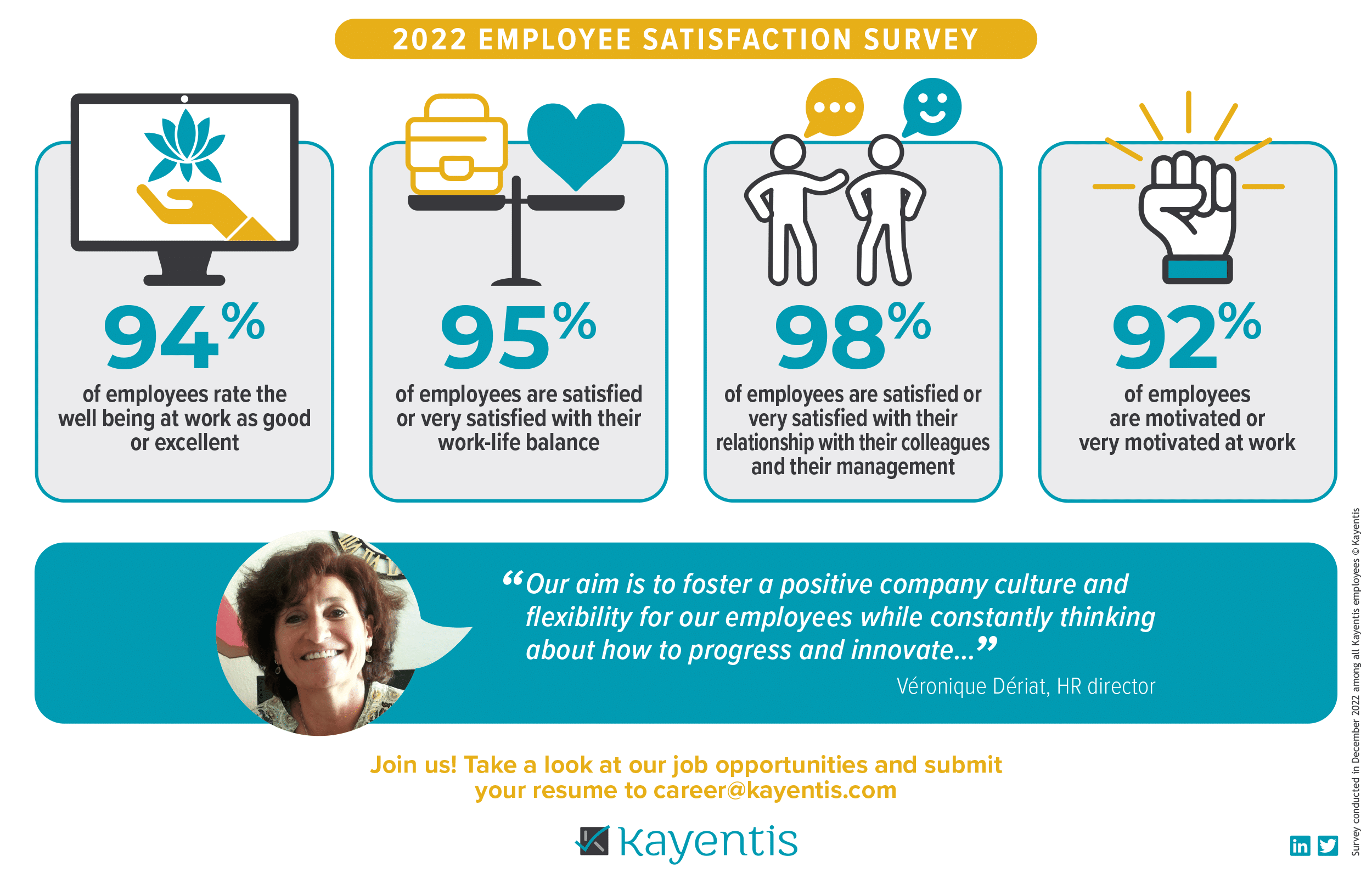
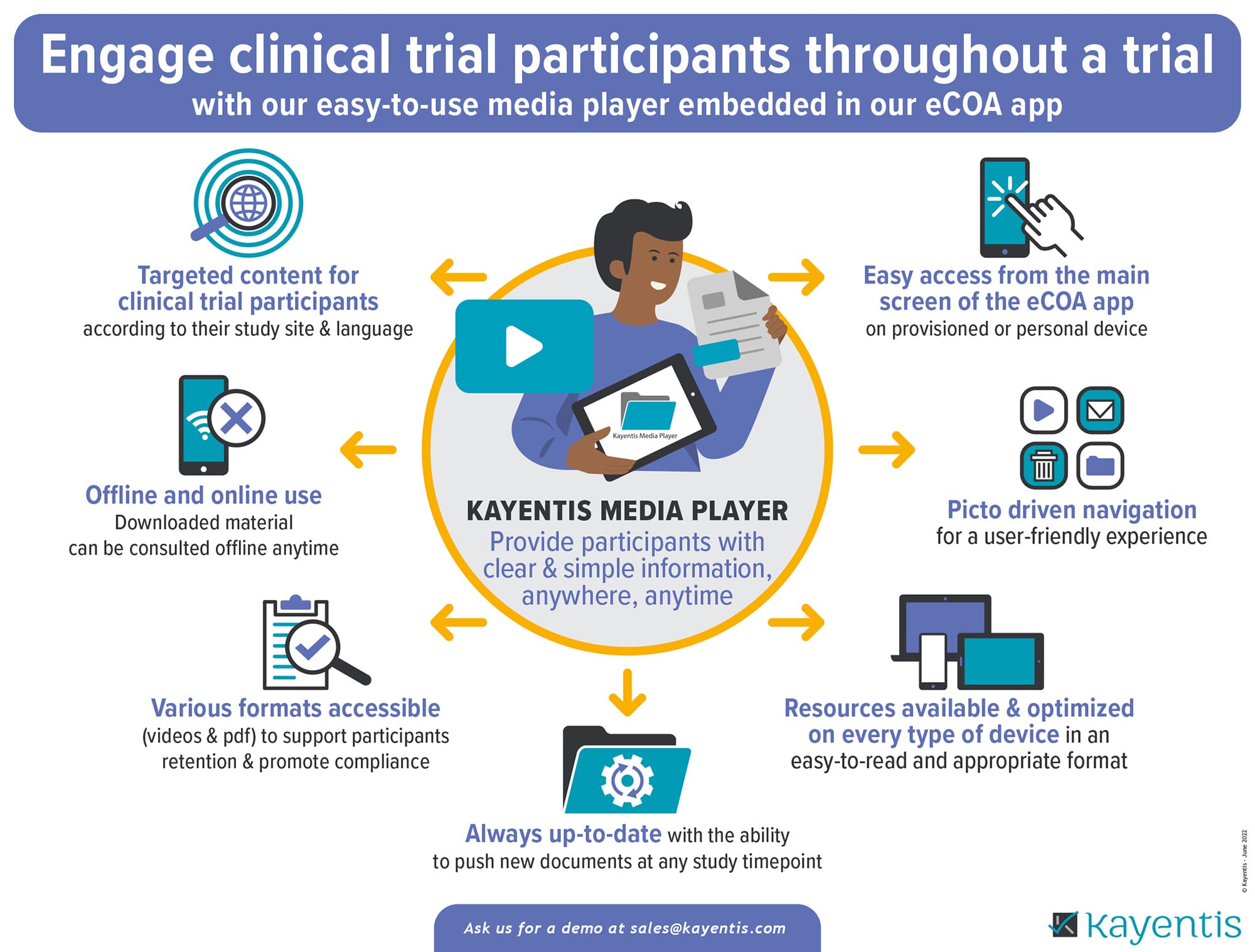
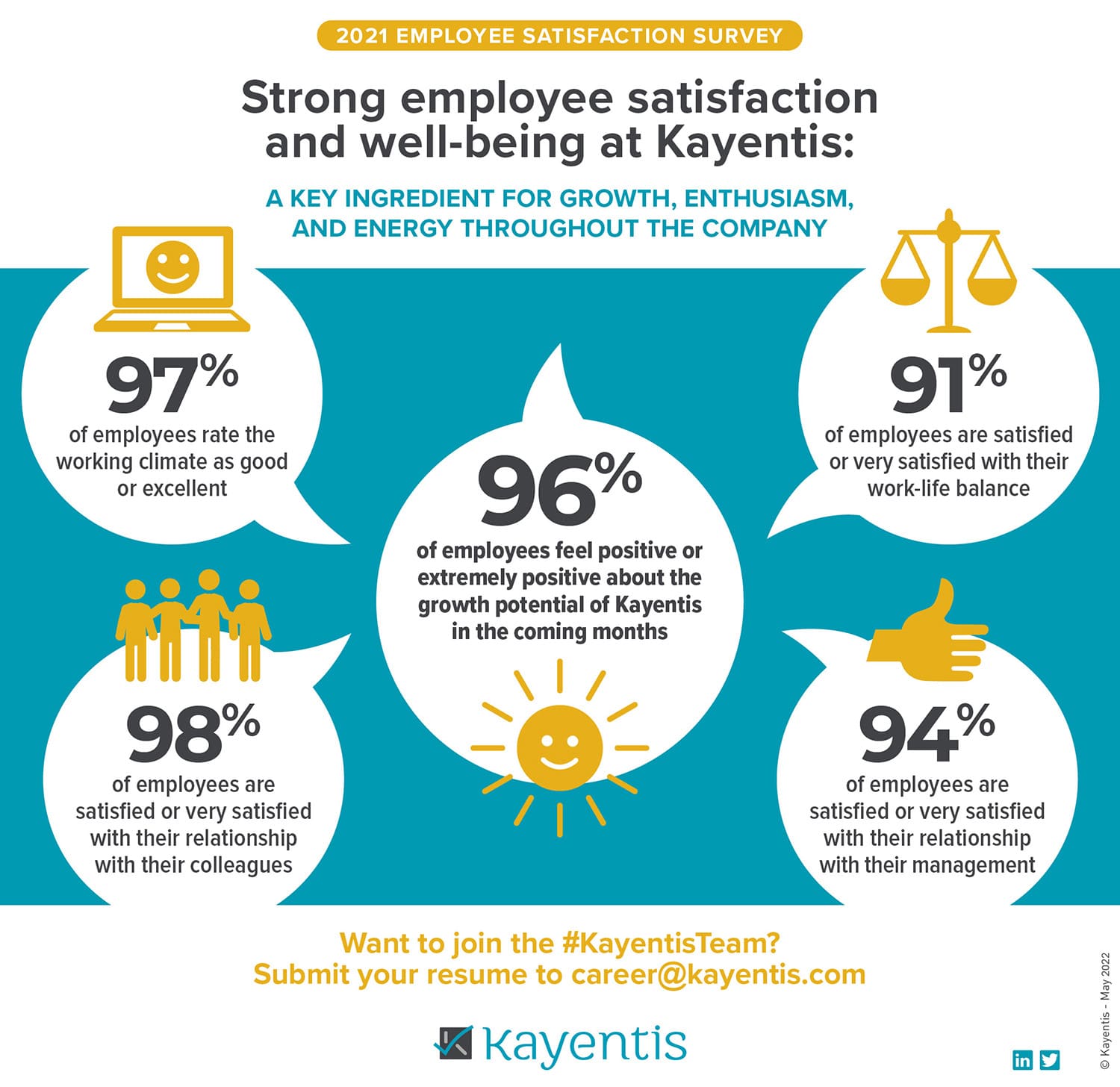
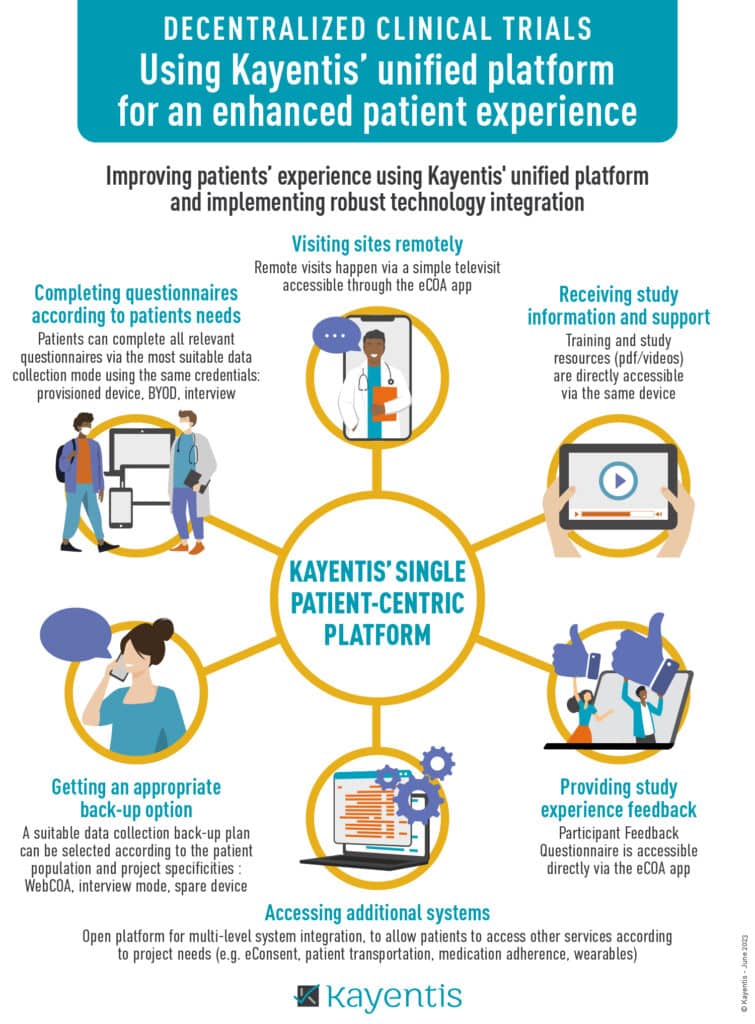
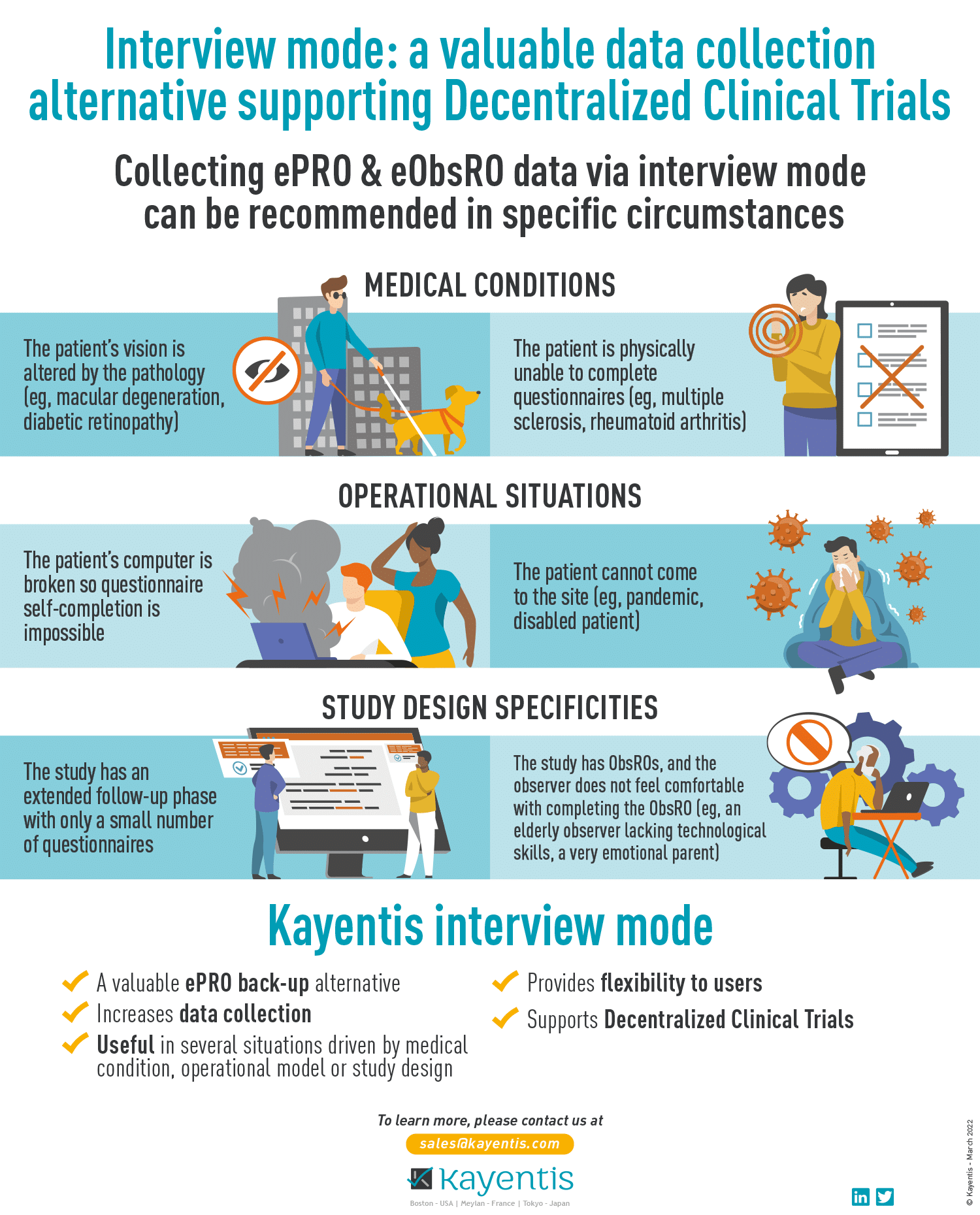
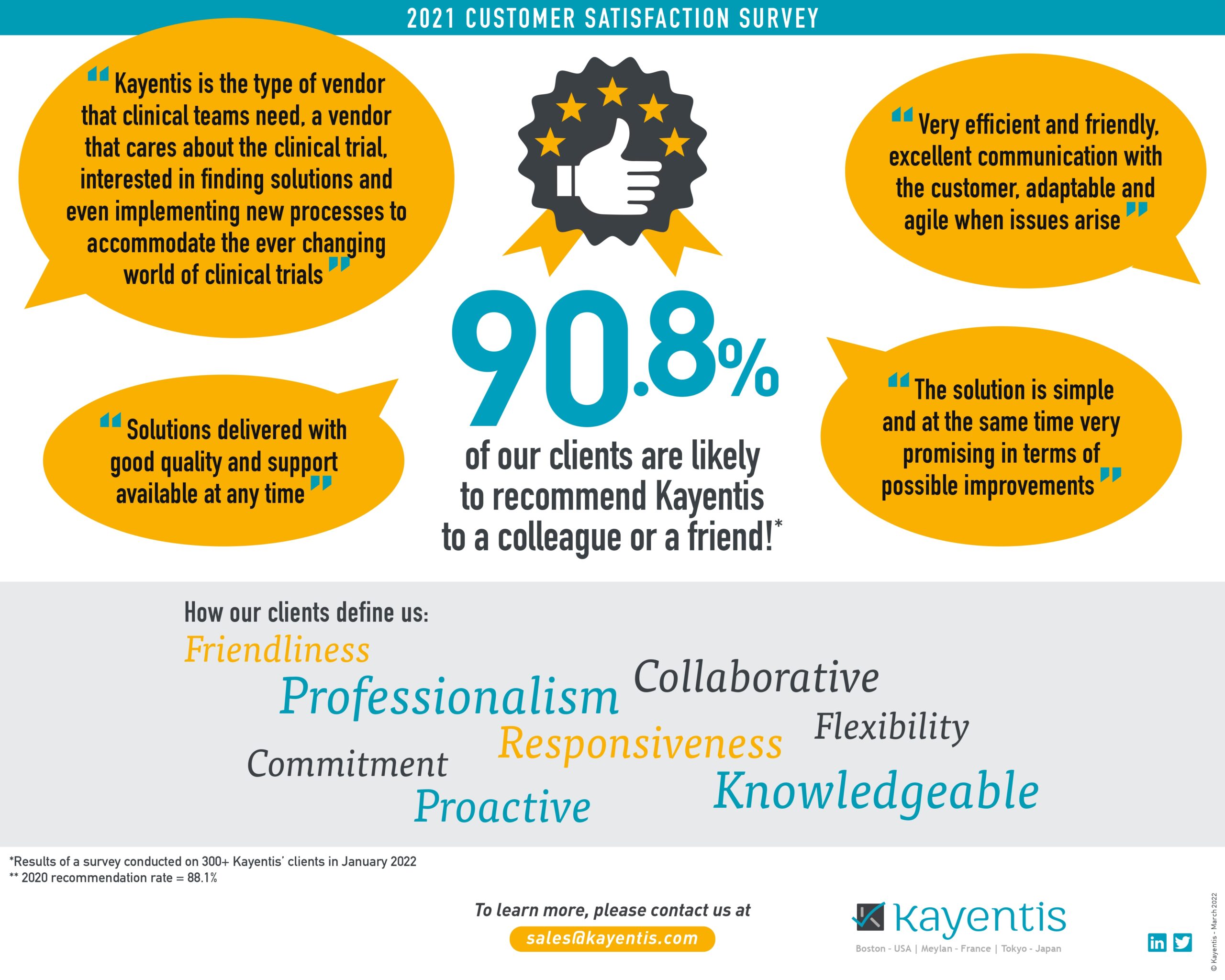
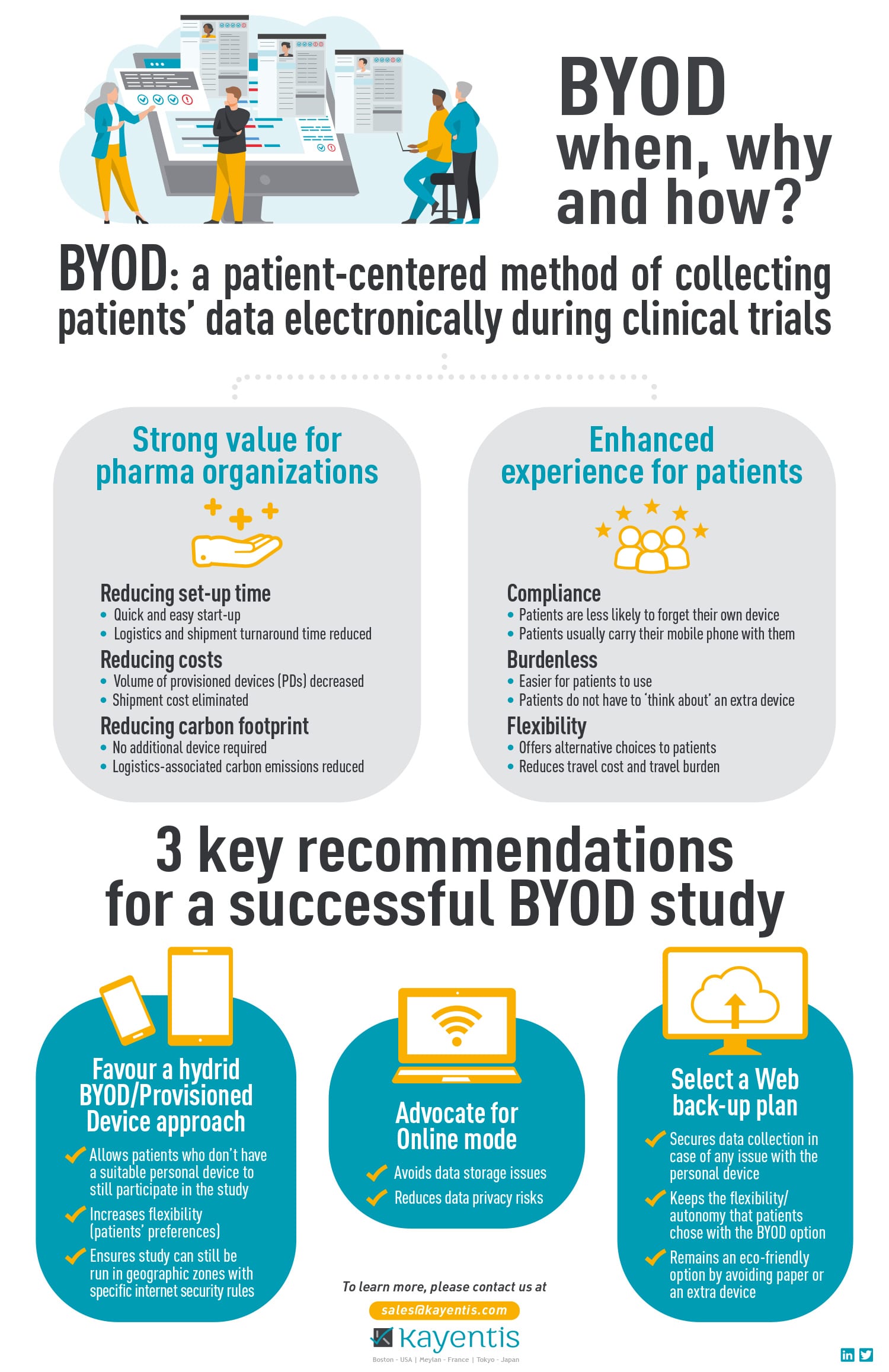
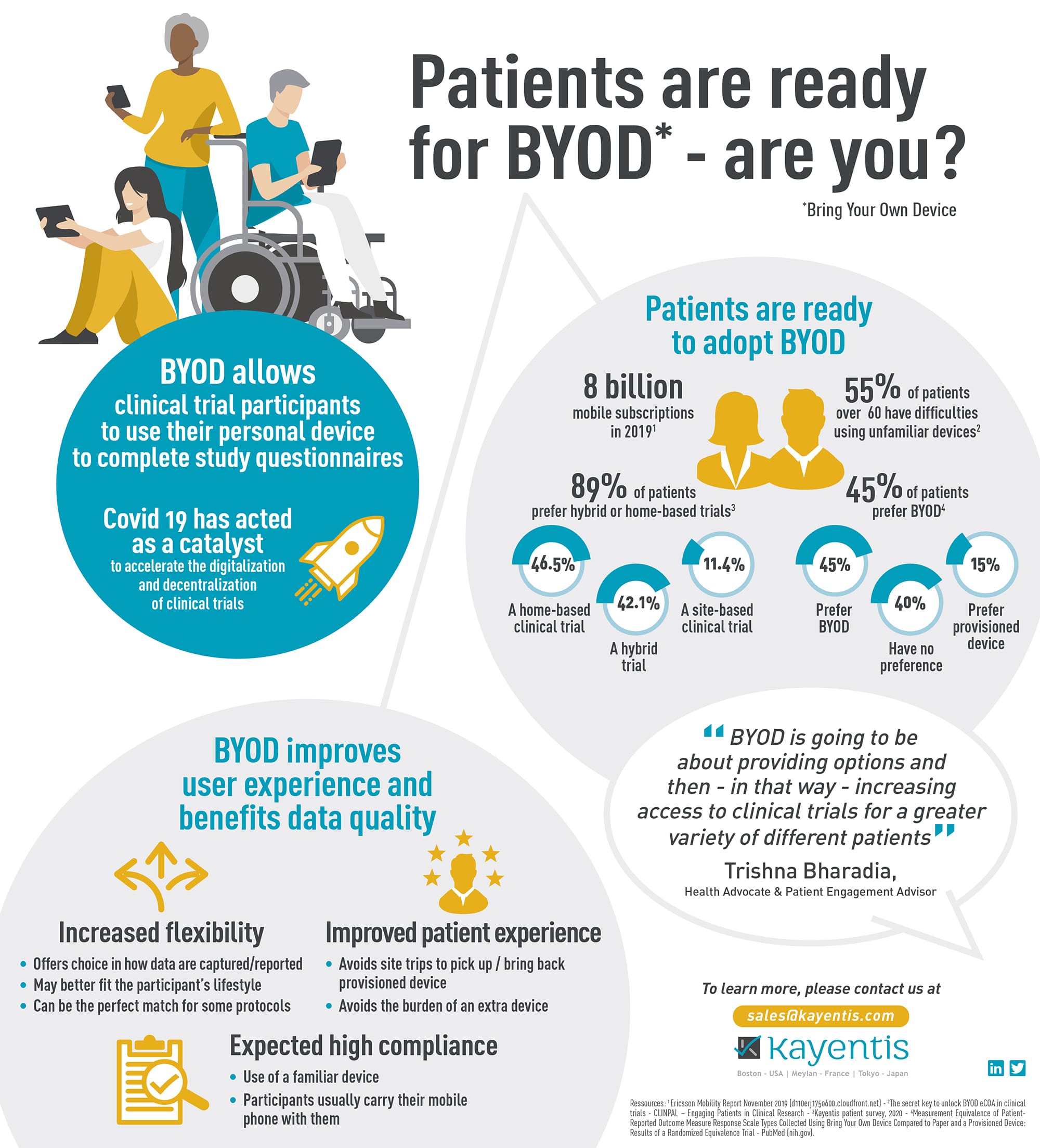
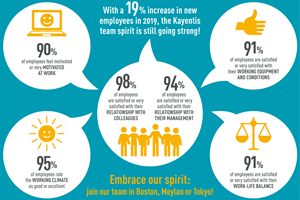
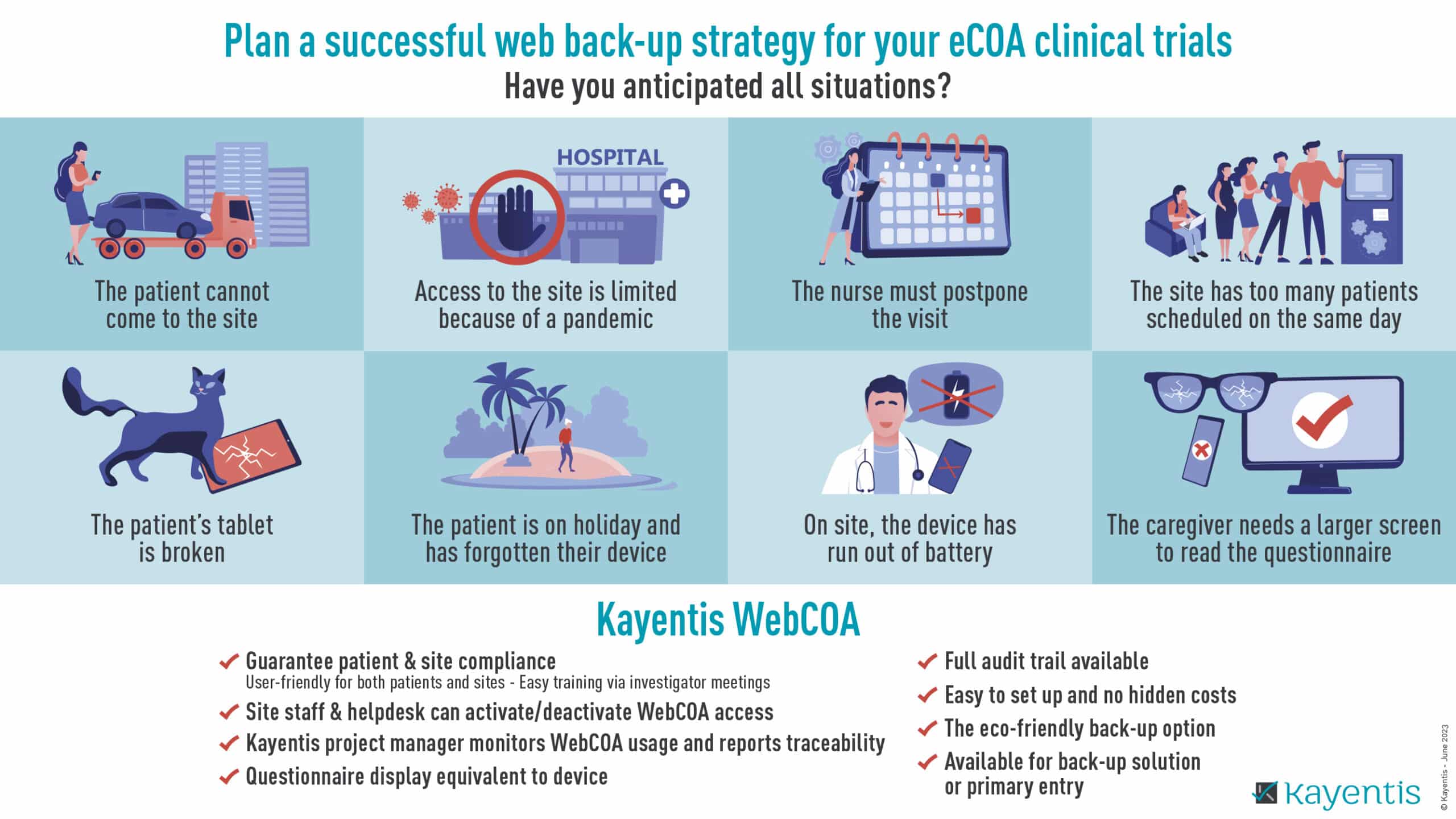
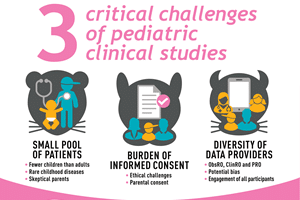
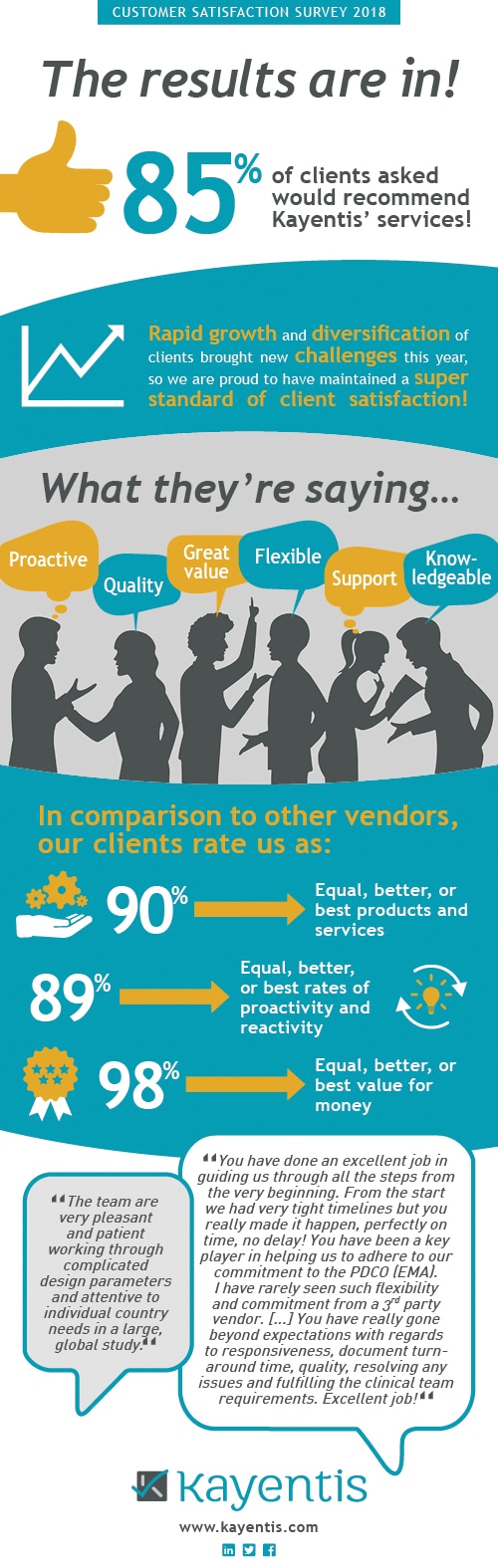
Infographic
Biotech and eCOA vendor: A shared mindset
Biotechs play a pivotal role in driving innovation, contributing significantly to the development of groundbreaking therapies for patients. But they are operating under tremendous pressure due to time and cost...