Webcast
Navigating BYOD in Clinical Research: Insights for Clinical Trial Success
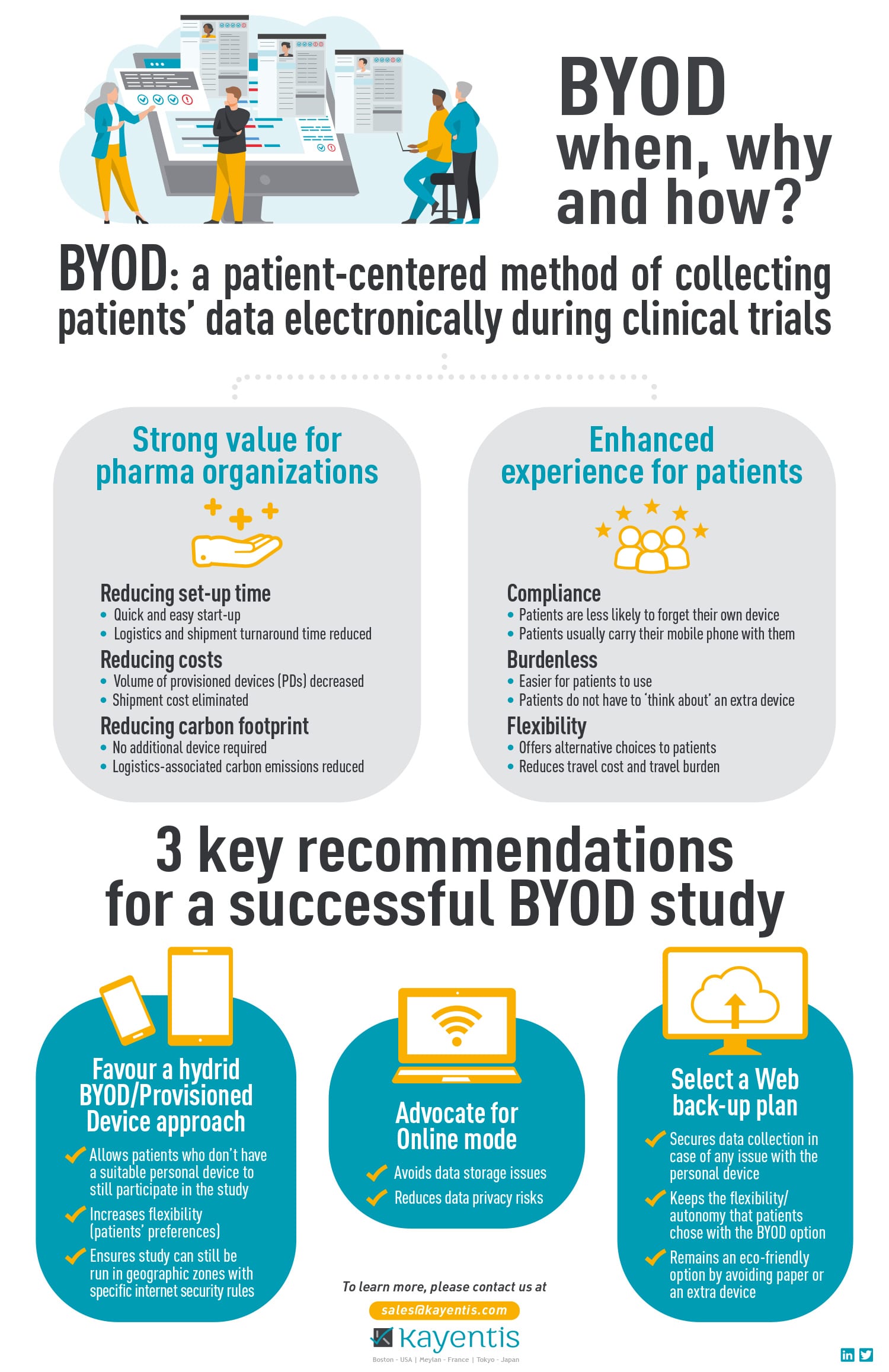
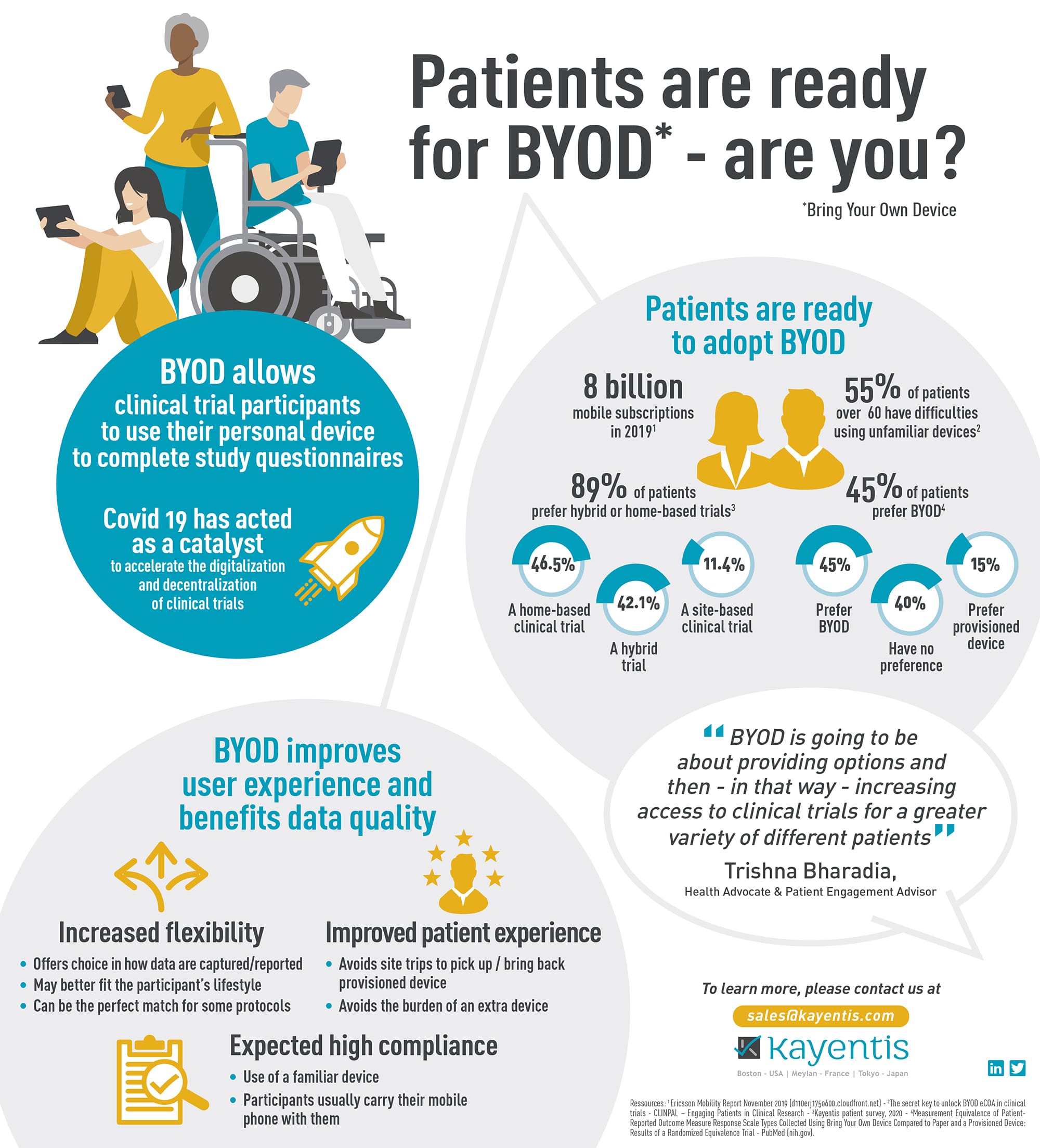
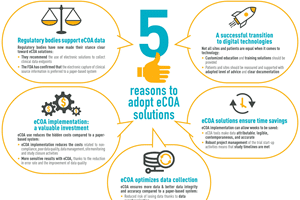
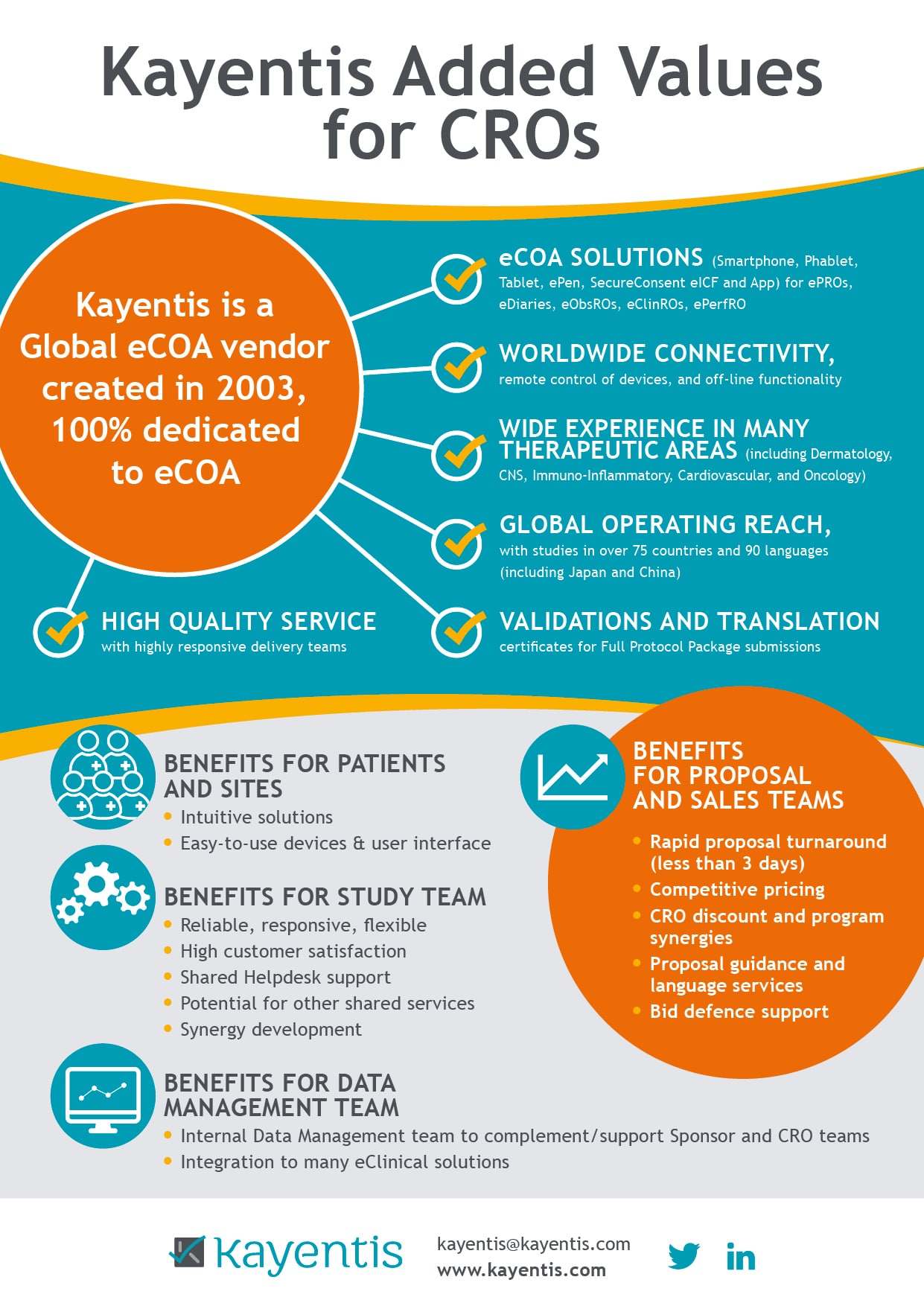
As the Bring Your Own Device (BYOD) model gains momentum in clinical research, understanding what to consider and how to implement it effectively for electronic Clinical Outcome Assessment (eCOA) data...